Synthesis of sodium polyacrylate–bentonite using in situ polymerization for Pb2+ removal from aqueous solutions
Youjun He,
Meishan Pei*,
Ni Xue,
Luyan Wang and
Wenjuan Guo
School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250000, China. E-mail: chm_peims@ujn.edu.cn; Tel: +86-531-82765394
First published on 5th May 2016
Abstract
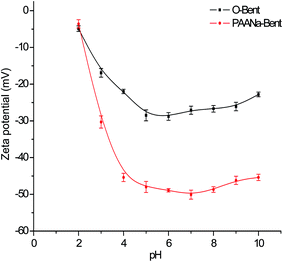
Polymer/clay composites have attracted a great deal of interest because of their wide applications in environmental protection. In this study, a sodium polyacrylate–bentonite material (PAANa–Bent), as an adsorbent for heavy metal ions, is synthesized for the first time using in situ polymerization. A series of analytical methods including FTIR, zeta potential measurements, SEM, TG/DTA, DSC, a N2 adsorption–desorption isotherm study and XRD were used to characterize PAANa–Bent. Furthermore, to evaluate the heavy metal ion adsorption capability of PAANa–Bent, batch experiments were conducted using Pb2+ as an adsorbate. The results demonstrate that PAANa–Bent was prepared successfully and the layered structure of bentonite was protected during polymerization. PAANa–Bent has more negative surface charges and a better dispersity than the original bentonite (O-Bent). The maximum adsorption capacity of PAANa–Bent is 70.41 mg g−1 at pH = 6, t = 300 min and T = 25 °C, an increase of 113% compared with O-Bent. A kinetic study shows that the adsorption process of PAANa–Bent obeys a pseudo-second-order model and the best-fit adsorption isotherm is a Freundlich isotherm, indicating the heterogeneous nature of PAANa–Bent. Besides, desorption experiments suggest that the PAANa–Bent can be regenerated easily with 0.1 M HCl as a stripping agent and the desorption ratio was still more than 91% after four cycles. Overall, the results indicate the potential of PAANa–Bent as a low-cost and highly efficient adsorbent for Pb2+ removal from aqueous solutions.
1. Introduction
Contamination with heavy metal ions is one source of environmental pollution and heavy metal ions are hazardous even at very low concentration. Heavy metal ions come from various industrial sources and are harmful to human health because they are non-biodegradable and bio-accumulate in the body.1 For example, excessive Cu2+ will cause a metallic taste in the mouth, stomach ulcers and mental retardation.2 Cr6+ will cause severe diarrhea, pulmonary congestion, and liver and kidney damage.3 The toxic effects of Ni include bone, nose and lung cancer, chest pains and so on.4 Pb2+ can’t be metabolized by the body and eventually is a cumulative poison, which damages the kidneys, liver, reproductive system, basic cellular processes and brain function. Pb2+ is especially hazardous to fetuses, babies and young children, as it impedes their normal mental and physical development.5,6 Therefore, the elimination of heavy metals from water is very important for public health and extensive research has been carried out concerning heavy metal ion removal from contaminated water.7Currently, the main methods used to remove heavy metal ions from water include chemical precipitation and filtration8 (hydroxides, sulfides, etc.), membrane technologies9–11 (reverse osmosis, nanofiltration, etc.), electrolysis,12–14 adsorption15,16 and ion-exchange.17,18 Among these techniques, adsorption has been proven to be an economical and effective method, owing to the flexibility in design and operation, high efficiency and low cost.19,20 In addition, the used adsorbent can be regenerated by a simple desorption process.21 The main adsorbents employed for heavy metal ion adsorption include activated carbon,22 synthetic polymers23 and silica-based adsorbents,24 though these adsorbents haven’t been widely employed in most industries due to developing nations being affected by their high cost.2
In recent years, bentonite has attracted enormous attention due to its abundance, low cost,25–27 and unique cation exchange capacity (CEC), arising from isomorphous substitution (e.g., Mg2+ for Al3+ in the octahedral sheet, or Fe3+ or Al3+ for Si4+ in the tetrahedral sheet) in the montmorillonite layers.28 Bentonite is a clay-based natural material and the major component is mineral montmorillonite, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered clay consisting of an octahedral alumina (AlO6) sheet sandwiched between two tetrahedral silica (SiO4) sheets.29,30 There are numerous terminal –OH groups on the crystal edges of the aluminate sheets, which provide anchoring sites for modification.31 Thereby, these advantages make bentonite a widely used adsorbent in the field of environmental remediation, such as in the fate and transport of heavy metal ions,32,33 dyes34,35 and other inorganic or organic pollutants.36,37 However, the original bentonite always presents some drawbacks such as the aggregation of bentonite particles and low adsorption efficiency.38,39 Therefore, some modifications have been performed to improve the dispersibility and adsorption capacity of bentonite. Recently, the main modifications of bentonite have included forming (i) pillared clays,40 (ii) organoclays,41,42 and (iii) polymer/clay composites.43 Among all the approaches, the use of polymer/clay composites has been found to be one of the most effective methods, owing to a variety of possible polymerization methods, the controllable degree of the polymerization and the numerous functional groups present in the molecular chains.44,45 For example, Emmanuel I. Unuabonah et al.46 prepared a polyvinyl alcohol–clay composite used to remove Pb2+ from an aqueous solution; Xiaohuan Wang et al.47 prepared a novel chitosan–poly(vinyl alcohol)/bentonite (CTS–PVA/BT) composite for adsorption of Hg(II) and the CTS–PVA/BT showed a high adsorption capacity for Hg(II); Li et al.48 prepared a cationic-polymer/bentonite (EPI-DMA/bentonite) composite for the removal of non-ionic and anionic dyes. However these traditional polymer/clay composites are unstable and unbind, because the bonding mechanism of the polymer/clay composites is only physical adsorption between the polymer and clay.49,50 In addition, the layered structure of bentonite will be destroyed due to polymerization of monomers in the interlayers.51 To date, there isn’t yet a report about the modification of clay using polymers, through covalent bonds, where at the same time the layered structure of bentonite is protected.
1 layered clay consisting of an octahedral alumina (AlO6) sheet sandwiched between two tetrahedral silica (SiO4) sheets.29,30 There are numerous terminal –OH groups on the crystal edges of the aluminate sheets, which provide anchoring sites for modification.31 Thereby, these advantages make bentonite a widely used adsorbent in the field of environmental remediation, such as in the fate and transport of heavy metal ions,32,33 dyes34,35 and other inorganic or organic pollutants.36,37 However, the original bentonite always presents some drawbacks such as the aggregation of bentonite particles and low adsorption efficiency.38,39 Therefore, some modifications have been performed to improve the dispersibility and adsorption capacity of bentonite. Recently, the main modifications of bentonite have included forming (i) pillared clays,40 (ii) organoclays,41,42 and (iii) polymer/clay composites.43 Among all the approaches, the use of polymer/clay composites has been found to be one of the most effective methods, owing to a variety of possible polymerization methods, the controllable degree of the polymerization and the numerous functional groups present in the molecular chains.44,45 For example, Emmanuel I. Unuabonah et al.46 prepared a polyvinyl alcohol–clay composite used to remove Pb2+ from an aqueous solution; Xiaohuan Wang et al.47 prepared a novel chitosan–poly(vinyl alcohol)/bentonite (CTS–PVA/BT) composite for adsorption of Hg(II) and the CTS–PVA/BT showed a high adsorption capacity for Hg(II); Li et al.48 prepared a cationic-polymer/bentonite (EPI-DMA/bentonite) composite for the removal of non-ionic and anionic dyes. However these traditional polymer/clay composites are unstable and unbind, because the bonding mechanism of the polymer/clay composites is only physical adsorption between the polymer and clay.49,50 In addition, the layered structure of bentonite will be destroyed due to polymerization of monomers in the interlayers.51 To date, there isn’t yet a report about the modification of clay using polymers, through covalent bonds, where at the same time the layered structure of bentonite is protected.
In this work, in order to prepare a layered covalently-bonded polymer–clay with a high adsorption capability for heavy metal ions, a sodium polyacrylate–bentonite (PAANa–Bent) material is synthesized for the first time using in situ polymerization on the surface of the bentonite through covalent bonds. The use of sodium acrylate can efficiently avoid the structural damage to bentonite caused by polymerization in the interlayers because sodium acrylate is an anion in water and this will stop it entering into the electronegative interlayer spaces arising from isomorphous substitution. Meanwhile, the large number of carboxyl groups provides new adsorption sites for Pb2+. The prepared sodium polyacrylate–bentonite is characterized using Fourier transform infrared spectroscopy (FTIR), zeta potential measurements, scanning electron microscopy,51 thermogravimetric analysis (TG/DTA), differential scanning calorimetry (DSC), N2 adsorption/desorption experiments and X-ray diffraction (XRD). Lastly, Pb2+ is chosen as an adsorbate to evaluate the heavy metal ion adsorption capability of PAANa–Bent and the adsorption mechanism of Pb2+ with PAANa–Bent is also investigated.
2. Experimental
2.1. Materials and chemicals
The original bentonite (O-Bent) used in this study was purchased from Weifang Huawei Bentonite Co., Ltd. (Weifang, Shandong Province, China). (3-Aminopropyl)trimethoxysilane (APTS), acryloyl chloride, triethylamine, sodium acrylate and potassium persulfate (K2S2O8) were all purchased from Aladdin Industrial Corporation. Pb(NO3)2, used as a Pb2+ source, was provided by Sinopharm Chemical Reagent Co., Ltd (China). All chemicals were analytical grade and used as-received. Deionized water was used in the preparation of all the solutions.2.2. Preparation of sodium polyacrylate–bentonite
The synthetic route is reported in Fig. 1. Firstly, APTS (2 g) dissolved in 200 mL of ethyl alcohol/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was anchored onto bentonite (4 g) over 8 h at 60 °C and the resulting intermediate was filtered, washed with deionized water and dried at 60 °C under vacuum (denoted as NH2–Bent). Next, 3 g of NH2–Bent and 2.5 g of triethylamine were combined in 100 mL of CH2Cl2 and the mixture was cooled to 0 °C. To this solution, 7 mL of acryloyl chloride diluted in 100 mL of CH2Cl2 was added dropwise, under cooling in an ice bath. The reaction was allowed to warm to room temperature overnight. After being filtered and washed with CH2Cl2, the product was designated as CH2
1, v/v) was anchored onto bentonite (4 g) over 8 h at 60 °C and the resulting intermediate was filtered, washed with deionized water and dried at 60 °C under vacuum (denoted as NH2–Bent). Next, 3 g of NH2–Bent and 2.5 g of triethylamine were combined in 100 mL of CH2Cl2 and the mixture was cooled to 0 °C. To this solution, 7 mL of acryloyl chloride diluted in 100 mL of CH2Cl2 was added dropwise, under cooling in an ice bath. The reaction was allowed to warm to room temperature overnight. After being filtered and washed with CH2Cl2, the product was designated as CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Bent. The bentonite precursor with reactive carbon–carbon double bonds had been prepared. Then, 1 g of CH2
CH–Bent. The bentonite precursor with reactive carbon–carbon double bonds had been prepared. Then, 1 g of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Bent and 0.8 g of K2S2O8 were dispersed in 100 mL of deionized water and a 95 mL aqueous solution of sodium acrylate (5 wt%) was added dropwise. The polymerization reaction was conducted for 4 h at 60 °C under a N2 atmosphere. The product was filtered and washed with a Soxhlet extractor to remove any sodium polyacrylate that wasn’t bonded to the surface of the bentonite. The resultant product was named PAANa–Bent, after it was vacuum dried at 60 °C and ground.
CH–Bent and 0.8 g of K2S2O8 were dispersed in 100 mL of deionized water and a 95 mL aqueous solution of sodium acrylate (5 wt%) was added dropwise. The polymerization reaction was conducted for 4 h at 60 °C under a N2 atmosphere. The product was filtered and washed with a Soxhlet extractor to remove any sodium polyacrylate that wasn’t bonded to the surface of the bentonite. The resultant product was named PAANa–Bent, after it was vacuum dried at 60 °C and ground.
2.3. Characterization
2.4. Batch adsorption experiments
The adsorption of Pb2+ onto O-Bent and PAANa–Bent was studied using batch adsorption experiments. The effects of dominating experimental variables, including the contact time (t), pH and initial concentration (ci) of Pb2+, were determined. The effect of the contact time was studied for a range of 30–720 min by adding 0.05 g of O-Bent or PAANa–Bent to 40 mL of a Pb2+ solution, at a solution concentration of 100 mg L−1, pH = 6 and at a room temperature of 25 °C. The effect of pH was determined for pH values of 2–6 by adding 0.05 g of O-Bent or PAANa–Bent to 40 mL of a Pb2+ solution, at a solution concentration of 100 mg L−1, contact time of 300 min and at a room temperature of 25 °C. The effect of the initial Pb2+ concentration was determined for a concentration range of 20–300 mg L−1 by adding 0.05 g of O-Bent or PAANa–Bent to 40 mL of a Pb2+ solution, with a contact time of 300 min, pH = 6 and at a room temperature of 25 °C. The detailed batch adsorption experimental conditions are listed in Table 1.| Adsorbent | t (min) | pH | ci (mg L−1) | ma (g) | Vs (mL) | T (°C) | S (rpm) |
|---|---|---|---|---|---|---|---|
| PAANa–Bent | 30–720 | 6 | 100 | 0.05 | 40 | 25 | 270 |
| (O-Bent) | 300 | 2–6 | 100 | 0.05 | 40 | 25 | 270 |
| 300 | 6 | 20–300 | 0.05 | 40 | 25 | 270 |
Each experiment was performed in triplicate and the mean values were computed to ensure quality assurance. All batch adsorption experiments were conducted in a temperature controlled water bath shaker (Dong Peng SHA-C). The adsorbed amount of Pb2+ with PAANa–Bent was calculated using the following equation:
 | (1) |
3. Results and discussion
3.1. Characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Bent and PAANa–Bent display a typical ν–OH (Al–OH) at 3632 cm−1 and ν–OH (H–OH) at 3428 cm−1.52 The band at 1638 cm−1 corresponds to the deformation vibration of H–OH.53 The intensive band around 1040 cm−1 is attributed to νSi–O. The band at 920 cm−1 represents νAl–O.54 The peak at 798 cm−1 is a result of Fe–O stretching vibrations.55 The deformation of Si–O appears at 624 cm−1.56 The bands at 524 cm−1 are ascribed to the Si–O–Al and Si–O–Si bending vibrations.57 The bands of NH2–Bent, CH2
CH–Bent and PAANa–Bent display a typical ν–OH (Al–OH) at 3632 cm−1 and ν–OH (H–OH) at 3428 cm−1.52 The band at 1638 cm−1 corresponds to the deformation vibration of H–OH.53 The intensive band around 1040 cm−1 is attributed to νSi–O. The band at 920 cm−1 represents νAl–O.54 The peak at 798 cm−1 is a result of Fe–O stretching vibrations.55 The deformation of Si–O appears at 624 cm−1.56 The bands at 524 cm−1 are ascribed to the Si–O–Al and Si–O–Si bending vibrations.57 The bands of NH2–Bent, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Bent and PAANa–Bent at 2935 cm−1 correspond to the asymmetric stretching of –CH2– after modification. The band at 3290 cm−1 of CH2
CH–Bent and PAANa–Bent at 2935 cm−1 correspond to the asymmetric stretching of –CH2– after modification. The band at 3290 cm−1 of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Bent is caused by C
CH–Bent is caused by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching vibrations. The spectra of PAANa–Bent with bands at 1590 and 1409 cm−1 are indicative of COO− asymmetric and symmetric stretching, respectively.
C–H stretching vibrations. The spectra of PAANa–Bent with bands at 1590 and 1409 cm−1 are indicative of COO− asymmetric and symmetric stretching, respectively.
| Sample | O-Bent | PAANa–Bent |
|---|---|---|
| SBET (m2 g−1) | 16.337 ± 0.042 | 5.891 ± 0.096 |
| V0.99 (cm3 g−1) | 0.063 ± 8.165 × 10−4 | 0.030 ± 3.37 × 10−4 |
| Vmicro (cm3 g−1) | 0.005 ± 1.715 × 10−5 | 0.000 |
| Vmeso (cm3 g−1) | 0.062 ± 5.307 × 10−4 | 0.029 ± 7.92 × 10−5 |
3.2. Adsorption study of Pb2+
 | (2) |
Eqn (2) was integrated with the boundaries of t = 0 to t = t and q = 0 to q = q, giving
 | (3) |
For the pseudo-second-order model, it is considered that the adsorption rate is proportional to the square of the number of unoccupied adsorption sites, and the rate equation is
 | (4) |
Eqn (4) was integrated with the boundaries of t = 0 to t = t and q = 0 to q = q, giving
 | (5) |
The values of the calculated kinetic data are shown in Fig. 10 and listed in Table 3, together with the corresponding correlation coefficients R2 and experimental equilibrium amounts qexpe. Compared with the pseudo-first-order model, the pseudo-second-order model with higher correlation coefficients R2 is considered more appropriate for depicting the adsorption processes of O-Bent and PAANa–Bent. Besides, qexpe and qe obtained from the pseudo-second model show excellent agreement.
| Adsorbent | qexpe (mg g−1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (min−1) | R2 | qe (mg g−1) | k2 (g mg−1 min−1) | R2 | ||
| O-Bent | 33.07 | 14.43 | 7.28 × 10−2 | 0.83 | 33.47 | 4.63 × 10−3 | 0.99 |
| PAANa–Bent | 70.41 | 143.71 | 4.17 × 10−3 | 0.97 | 70.87 | 1.94 × 10−3 | 0.99 |
 | (6) |
 | (7) |
The linear simulations are shown in Fig. 14 and their parameters are listed in Table 4. In the case of O-Bent, the adsorption data fit well to the Langmuir adsorption isotherm compared to the Freundlich adsorption isotherm, based on the higher correlation coefficient R2. In addition, the calculated qm (37.23 mg g−1) is in agreement with the qexpm (33.07 mg g−1). This suggests that a monolayer adsorption of Pb2+ takes place and that there is only one type of adsorption site in O-Bent, i.e. a cation exchange capacity in the interlayers. However, the Freundlich adsorption isotherm is more suitable for PAANa–Bent, according to the higher correlation coefficient R2. This confirms heterogeneous surfaces (more than one type of binding site) of PAANa–Bent after modification. This is because there are two types of adsorption sites coexisting in PAANa–Bent, i.e. –COO− groups on the surface of PAANa–Bent and a cation exchange capacity in the interlayers. The high KF (16.80) indicates a high adsorption capability and that adsorption takes place on heterogeneous surfaces.59,61 The value of n (1.68) lies between 1 and 10, indicating a favorable adsorption of Pb2+ using PAANa–Bent.1
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (L mg−1) | qm (mg L−1) | R2 | KF (mg g−1) | n (L mg−1) | R2 | |
| O-Bent | 0.17 | 37.23 | 0.99 | 10.75 | 3.33 | 0.94 |
| PAANa–Bent | 0.18 | 94.61 | 0.91 | 16.80 | 1.68 | 0.98 |
3.3. Regeneration
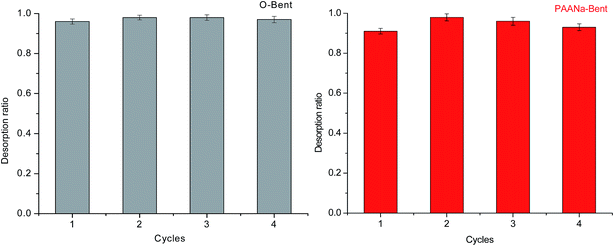
The reuse of adsorbents helps to minimize the cost of the adsorption process. Thus, for an efficient adsorbent, a good desorption potential is important. Desorption experiments were performed with a 0.1 M HCl solution as the stripping agent. Firstly, 0.05 g of O-Bent or PAANa–Bent was added into 40 mL of a Pb2+ aqueous solution (100 mg L−1) and shaken for 300 min at 25 °C, pH = 6. After the first adsorption, the adsorbent was regenerated using 100 mL of 0.1 M HCl for 2 h at 25 °C. The adsorbent after elution was washed to neutral with deionized water and used for the next adsorption cycle. The adsorption–desorption cycle was repeated 4 times to assess the reusability of O-Bent and PAANa–Bent. The desorption experiments were performed in triplicate and the mean values are presented. The desorption ratio was calculated using the equation:| Desorption ratio = VDCD/qe | (8) |
Fig. 15 shows the performance of O-Bent and PAANa–Bent after regeneration. As observed, O-Bent shows an excellent reusability and the desorption ratios for the four cycles are all higher than 96%. The decline in the desorption ratio may be due to the fact that there is still a small amount of Pb2+ retained in the adsorbent after the desorption process. Compared to O-Bent, the reusability of PAANa–Bent declined slightly, however the desorption ratios for the four cycles are all more than 91%. The slight decrease may be due to more adsorption sites of PAANa–Bent to impede Pb2+ desorption. In general, the desorption experiments confirmed that PAANa–Bent is a recyclable adsorbent and can be easily regenerated with 0.1 M HCl.
4. Conclusions
In conclusion, PAANa–Bent as a novel adsorbent was prepared successfully using in situ polymerization on the surface of bentonite. A series of analytical methods were applied to characterize the PAANa–Bent. Having the sodium polyacrylate anchored on the surface of the bentonite through covalent bonds is different from the traditional polymer/clay composites formed by physical adsorption. In addition, the layered structure of bentonite was protected from polymerization due to the selection of sodium acrylate as the monomer.Compared to the maximum qe (33.07 mg g−1) of O-Bent, the maximum qe of PAANa–Bent of 70.41 mg g−1 had increased by approximately 113% under optimum conditions (pH = 6, t = 300 min ci = 100 mg L−1 and T = 25 °C). This indicates that modification causes a significant improvement of the Pb2+ adsorption capacity of bentonite.
The kinetic adsorption data of PAANa–Bent follow a pseudo-second-order model. Besides, the adsorption isotherm data of PAANa–Bent are best fitted with a Freundlich isotherm, revealing the heterogeneous nature of PAANa–Bent. The value of n lies between 1 and 10, indicating a favorable adsorption of Pb2+ using PAANa–Bent.
The high desorption ratio of Pb2+ from the PAANa–Bent suggests a good reusability.
Acknowledgements
Prof. Dr Bin Han (University of Jinan) and Dr Yue Wu (Qilu University of Technology) are acknowledged for help with the test instruments. Prof. Dr Wei (Lamar University) is acknowledged for help with the language.References
- K. G. Akpomie and F. A. Dawodu, Beni-Suef University Journal of Basic and Applied Sciences, 2015, 4, 1–13 CrossRef.
- R. M. Ali, H. A. Hamad, M. M. Hussein and G. F. Malash, Ecol. Eng., 2016, 91, 317–332 CrossRef.
- I. Ullah, A. Abbas, A. M. Al-Amer, T. Laoui, M. J. Al-Marri, M. S. Nasser, M. Khraisheh and M. A. Atieh, Sep. Purif. Technol., 2016, 157, 141–161 CrossRef.
- I. Mobasherpour, E. Salahi and M. Ebrahimi, Res. Chem. Intermed., 2012, 38, 2205–2222 CrossRef CAS.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- H. Hamad, Z. Ezzeddine, S. Kanaan, F. Lakis, A. Hijazi and M.-A. Moussawi, Adv. Powder Technol., 2016, 27, 631–637 CrossRef CAS.
- H.-L. Fan, L. Li, S.-F. Zhou and Y.-Z. Liu, Ceram. Int., 2016, 42, 4228–4237 CrossRef CAS.
- J. Khosravi and A. Alamdari, J. Hazard. Mater., 2009, 166, 695–700 CrossRef CAS PubMed.
- I. Petrinic, J. Korenak, D. Povodnik and C. Hélix-Nielsen, J. Cleaner Prod., 2015, 101, 292–300 CrossRef CAS.
- B. C. Ricci, C. D. Ferreira, A. O. Aguiar and M. C. S. Amaral, Sep. Purif. Technol., 2015, 154, 11–21 CrossRef CAS.
- P. Zhao, B. Gao, Q. Yue, S. Liu and H. K. Shon, Chem. Eng. J., 2016, 288, 569–576 CrossRef CAS.
- R.-h. Chen, L.-y. Chai, Y.-y. Wang, H. Liu, Y.-d. Shu and J. Zhao, Trans. Nonferrous Met. Soc. China, 2012, 22, 983–990 CrossRef CAS.
- B. Qin, H. Luo, G. Liu, R. Zhang, S. Chen, Y. Hou and Y. Luo, Bioresour. Technol., 2012, 121, 458–461 CrossRef CAS PubMed.
- L. Wu, L. Liao, G. Lv, F. Qin, Y. He and X. Wang, J. Hazard. Mater., 2013, 254–255, 277–283 CrossRef CAS PubMed.
- R. Khandanlou, H. R. Fard Masoumi, M. B. Ahmad, K. Shameli, M. Basri and K. Kalantari, Ecol. Eng., 2016, 91, 249–256 CrossRef.
- Z. Zarghami, A. Akbari, A. M. Latifi and M. A. Amani, Bioresour. Technol., 2016, 205, 230–238 CrossRef CAS PubMed.
- M. J. K. Ahmed and M. Ahmaruzzaman, Journal of Water Process Engineering, 2016, 10, 39–47 CrossRef.
- B. Sun, X. Li, R. Zhao, M. Yin, Z. Wang, Z. Jiang and C. Wang, J. Taiwan Inst. Chem. Eng., 2016, 62, 219–227 CrossRef CAS.
- M. A. Tofighy and T. Mohammadi, J. Hazard. Mater., 2011, 185, 140–147 CrossRef CAS PubMed.
- I. Ali, Chem. Rev., 2012, 112, 5073–5091 CrossRef CAS PubMed.
- B. Pan, B. Pan, W. Zhang, L. Lv, Q. Zhang and S. Zheng, Chem. Eng. J., 2009, 151, 19–29 CrossRef CAS.
- M. Karnib, A. Kabbani, H. Holail and Z. Olama, Energy Procedia, 2014, 50, 113–120 CrossRef CAS.
- C. Pires, A. P. G. C. Marques, A. Guerreiro, N. Magan and P. M. L. Castro, J. Hazard. Mater., 2011, 191, 277–286 CrossRef CAS PubMed.
- P. Wang, M. Du, H. Zhu, S. Bao, T. Yang and M. Zou, J. Hazard. Mater., 2015, 286, 533–544 CrossRef CAS PubMed.
- E. I. Unuabonah and A. Taubert, Appl. Clay Sci., 2014, 99, 83–92 CrossRef CAS.
- C. H. Zhou and J. Keeling, Appl. Clay Sci., 2013, 74, 3–9 CrossRef CAS.
- S. Hamidouche, O. Bouras, F. Zermane, B. Cheknane, M. Houari, J. Debord, M. Harel, J.-C. Bollinger and M. Baudu, Chem. Eng. J., 2015, 279, 964–972 CrossRef CAS.
- H. He, Y. Ma, J. Zhu, P. Yuan and Y. Qing, Appl. Clay Sci., 2010, 48, 67–72 CrossRef CAS.
- S. S. Metwally and R. R. Ayoub, Appl. Clay Sci., 2016, 126, 33–40 CrossRef CAS.
- J. B. Swadling, P. V. Coveney and H. C. Greenwell, J. Am. Chem. Soc., 2010, 132, 13750–13764 CrossRef CAS PubMed.
- S. Ng and J. Plank, Cem. Concr. Res., 2012, 42, 847–854 CrossRef CAS.
- M. L. Cantuaria, A. F. de Almeida Neto, E. S. Nascimento and M. G. A. Vieira, J. Cleaner Prod., 2016, 112, 1112–1121 CrossRef CAS.
- V. Masindi and W. M. Gitari, J. Cleaner Prod., 2016, 112, 1077–1085 CrossRef CAS.
- K. Chinoune, K. Bentaleb, Z. Bouberka, A. Nadim and U. Maschke, Appl. Clay Sci., 2016, 123, 64–75 CrossRef CAS.
- R. Huang, L. Zhang, P. Hu and J. Wang, Int. J. Biol. Macromol., 2016, 86, 496–504 CrossRef CAS PubMed.
- Y. Gao, Y. Guo and H. Zhang, J. Hazard. Mater., 2016, 302, 105–113 CrossRef CAS PubMed.
- F. Tomul, F. Turgut Basoglu and H. Canbay, Appl. Surf. Sci., 2016, 360, 579–593 CrossRef CAS.
- F. I. El-Dib, F. M. Tawfik, G. Eshaq, H. H. H. Hefni and A. E. ElMetwally, Int. J. Biol. Macromol., 2016, 86, 750–755 CrossRef CAS PubMed.
- H. Shao, X. Liu, Z. Zhou, B. Zhao, Z. Chen and M. Xu, Chem. Eng. J., 2016, 291, 306–316 CrossRef CAS.
- S. T. Khankhasaeva, D. V. Dambueva, E. T. Dashinamzhilova, A. Gil, M. A. Vicente and M. N. Timofeeva, J. Hazard. Mater., 2015, 293, 21–29 CrossRef CAS PubMed.
- D. A. D’Amico, R. P. Ollier, V. A. Alvarez, W. F. Schroeder and V. P. Cyras, Appl. Clay Sci., 2014, 99, 254–260 CrossRef.
- M. Kaya, K. Meral and Y. Onganer, J. Mol. Struct., 2015, 1083, 101–105 CrossRef CAS.
- I. Gardi, S. Nir and Y. G. Mishael, Water Res., 2015, 70, 64–73 CrossRef CAS PubMed.
- S. Kadi, S. Djadoun and N. Sbirrazzuoli, Thermochim. Acta, 2013, 569, 127–133 CrossRef CAS.
- A. M. Motawie, M. Madani, E. A. Esmail, A. Z. Dacrorry, H. M. Othman, M. M. Badr and D. E. Abulyazied, Egypt. J. Pet., 2014, 23, 379–387 CrossRef.
- E. I. Unuabonah, M. I. El-Khaiary, B. I. Olu-Owolabi and K. O. Adebowale, Chem. Eng. Res. Des., 2012, 90, 1105–1115 CrossRef CAS.
- X. Wang, L. Yang, J. Zhang, C. Wang and Q. Li, Chem. Eng. J., 2014, 251, 404–412 CrossRef CAS.
- Q. Li, Q. Y. Yue, H. J. Sun, Y. Su and B. Y. Gao, J. Environ. Manage., 2010, 91, 1601–1611 CrossRef CAS PubMed.
- Y. Deng, J. B. Dixon, G. N. White, R. H. Loeppert and A. S. R. Juo, Colloids Surf., A, 2006, 281, 82–91 CrossRef CAS.
- P. Liu, Appl. Clay Sci., 2007, 38, 64–76 CrossRef CAS.
- Y. Turhan, Z. G. Alp, M. Alkan and M. Doğan, Microporous Mesoporous Mater., 2013, 174, 144–153 CrossRef CAS.
- T. S. Anirudhan, P. S. Suchithra and S. Rijith, Colloids Surf., A, 2008, 326, 147–156 CrossRef CAS.
- N. Öztürk, A. Tabak, S. Akgöl and A. Denizli, Colloids Surf., A, 2008, 322, 148–154 CrossRef.
- P. Yuan, F. Annabi-Bergaya, Q. Tao, M. Fan, Z. Liu, J. Zhu, H. He and T. Chen, J. Colloid Interface Sci., 2008, 324, 142–149 CrossRef CAS PubMed.
- Z. X. Chen, X. Y. Jin, Z. Chen, M. Megharaj and R. Naidu, J. Colloid Interface Sci., 2011, 363, 601–607 CrossRef CAS PubMed.
- T. S. Anirudhan and M. Ramachandran, Ind. Eng. Chem. Res., 2008, 47, 6175–6184 CrossRef CAS.
- J. Guo, S. Chen, L. Liu, B. Li, P. Yang, L. Zhang and Y. Feng, J. Colloid Interface Sci., 2012, 382, 61–66 CrossRef CAS PubMed.
- F. Piscitelli, P. Posocco, R. Toth, M. Fermeglia, S. Pricl, G. Mensitieri and M. Lavorgna, J. Colloid Interface Sci., 2010, 351, 108–115 CrossRef CAS PubMed.
- M. Vhahangwele and G. W. Mugera, J. Environ. Chem. Eng., 2015, 3, 2416–2425 CrossRef CAS.
- D. Hamane, O. Arous, F. Kaouah, M. Trari, H. Kerdjoudj and Z. Bendjama, J. Environ. Chem. Eng., 2015, 3, 60–69 CrossRef CAS.
- R. Donat, A. Akdogan, E. Erdem and H. Cetisli, J. Colloid Interface Sci., 2005, 286, 43–52 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |