Prussian blue functionalized microcapsules for effective removal of cesium in a water environment†
Shanshan Fenga,
Xiaoda Lia,
Fang Maa,
Renfa Liub,
Guanglei Fua,
Sen Xinga and
Xiuli Yue*a
aState Key Laboratory of Urban Water Resources and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin, 150001, People's Republic of China. E-mail: xiulidx@163.com
bDepartment of Biomedical Engineering, College of Engineering, Peking University, Beijing 100871, People's Republic of China
First published on 30th March 2016
Abstract
In this work, a novel non-toxic and effective adsorbent, Prussian blue functionalized microcapsules (PB-MCs) was first developed for the highly efficient removal of cesium ions by flotation separation from aqueous solutions. The as-developed PB-MCs adsorbent exhibited excellent adsorption efficiency and rapid separation rate of cesium ions from aqueous solutions by floating due to the efficient combination of the advantages of poly(lactic acid) microcapsules (PLA MCs) and Prussian blue nanoparticles (PB NPs). Using different models for equilibrium and kinetic investigation, it was found that the goodness of mathematical fitting of the experimental data for the adsorption isotherms was in the order of Langmuir > Temkin > Freundlich, the kinetic models were in the order of pseudo-second-order > pseudo-first-order. The initial pH and temperature values both affected the adsorption efficiency of cesium ions. The improved adsorption and capacity of PB-MCs can be attributed to the anchoring technology, which prevented the aggregation of the microcapsules and thus increased the effective adsorption surface of the adsorbent. Furthermore, the PB-MCs adsorbent greatly reduced secondary environmental pollution because of the good biosafety of PB and completely biodegradable of PLA. Meanwhile, Prussian blue functionalized microcapsules could save energy consumption as a result of the flotation separation from wastewater after adsorption. Herein, such a functionalized PB-MCs adsorbent shows great potential applications for effective removal of cesium in wastewater treatment.
Introduction
Radioactive cesium (Cs) is one of the major fission products generated during the operation of most current nuclear power plants and has been involved in many of the nuclear accidents in the past.1–3 Such radioactive contaminants enter the food chain through plants and ultimately become incorporated into animals and human beings.4 As a result, removal of large amounts of radioactive cesium from the environment and nuclear waste is a subject of increased interest.2 Several adsorbents, such as zeolites,5 aluminum molybdophosphate,6 and polymers,7,8 have been developed to remove cesium ions from low-level radioactive water. Although most of these adsorbents achieved moderate removal efficiency at the primary research stages, they have to confront various limitations in the latter stage, which may limit their further large-scale applications.9 Meanwhile, the residual adsorbents in water may cause secondary environmental pollution. Therefore, developing new simple and efficient adsorbents for radioactive cesium removal is a research hotspot.Prussian blue (PB) is a prototype of mixed-valence transition metal hexacyanoferrates with the general formula of Fe4III[FeII(CN)6]3·nH2O. It has a face centered cubic lattice containing an open, zeolite-like structure. PB can be synthesized simply by mixing aqueous solutions of Fe3+ and [Fe(CN)6]4− and its low cost allows for large-scale application.10 Most importantly, PB is a typical U.S. FDA (USA Food and Drug Administration)-approved drug in clinic for treatment of radioactive exposure, demonstrating absolutely approved biosafety of PB in human body based on sufficient clinical trials.11 PB was used to treat Cs exposure in the Chernobyl nuclear reactor accident the Goiânia accident for its high ion-sieving functionality for Cs ions.12 However, direct application of PB for water treatment is not favorable since isolation of Cs-loaded PB from the water is a time- and money-consuming process.13 Therefore, a “green” and economic technique for PB immobilization and separation has attracted increasing interests.14–16
Poly(lactic acid) (PLA) is biodegradable aliphatic polyester industrially obtained from renewable resources, such corn or sugar beets.17 In recent years, as functional polymer materials, PLA has been applied in many fields, such as biomedical, food engineering, due to their significant biological and chemical properties like outstanding biocompatibility and biodegradability.18 Utilizing the facile water-in-oil-in-water (W/O/W) double-emulsion technique, the PLA could be engineered into bubble-containing microcapsules of various size, which have been widely studied for drug delivery and ultrasound imaging.19,20 The surface of PLA microcapsules (PLA MCs) could be easily loaded with various small molecules or nanoparticles via electrostatic layer-by-layer (LBL) self-assembly technique.21 Inspired by the phenomenon that after dispersed in water PLA MCs could float up to give a clear lower aqueous solution in a few minutes, we hypothesized that PLA MCs might be used for the immobilization and separation of PB NPs in the wastewater-treatment field.
Herein, we present a facile approach to synthesize functionalized Prussian blue microcapsules adsorbent for highly efficient removal of cesium ions by the flotation separation from aqueous solutions (Fig. 1). It demonstrates that PB NPs prepared with facile co-precipitation method was immobilized on the surface of PLA MCs to give PB NPs-loaded MCs (PB-MCs) for Cs+ removal. The PB nanocrystal monolayer of on the PLA MCs ensures enough contact area between PB with Cs-contaminated water, while the interior air bubbles of PLA MCs makes the separation process of Cs+-loaded PB NPs simple and efficient. Moreover, compared with the previous literature reports,13,22 this method shows dramatically combined something merits: (1) the PB-MCs adsorbent could be easily fabricated in a great quantity without a complicated reaction process, which is highly desired for real world application. (2) The PB-MCs adsorbent not only exhibited an obvious floating property associated with the simple flotation separation process but also showed a high adsorption capacity of cesium ions; in addition, all materials involved in PB-MCs adsorbent are biosafety and biodegradable, thus avoiding the problem of second environmental pollution. Meantime, the PB-MCs adsorbent can lower energy consumption, reduce cost and treat wastewater efficiently. Therefore, such Prussian blue functionalized microcapsules shows great promise for the purification of radiative Cs+-contaminated wastewater.
Experimental section
Materials and chemicals
Poly(lactic acid) (80k MW, Shandong Medical Instrumental Institute, China), polyvinyl alcohol (86–89% hydrolyzed, low molecular weight, Alfa Aesar), hydroxylamine hydrochloride (NH2OH·HCl, 1.5 mL, 40 mmol L−1, Shanghai Shanpu Chemical, China) were used as received. Branched polyethylene mine (PEI 800 MW) and camphor were purchased from Sigma-Aldrich. FeCl3·6H2O, K4[Fe(CN)6]·3H2O and citric acid were of analytical reagent grade and used as received. Cesium chloride (CsCl, 99.99%) was purchased from Merck. All reagents and solvents were of analytical grade and used as received without further purification. All aqueous solutions were prepared with deionized (DI) water (18.2 MΩ cm at 293 K, pH of 5.5) from a Milli-Q purification system.Synthesis of Prussian blue nanoparticles
PB NPs sized 45 nm was synthesized using previously-described method with some minor modifications.23 In this method, 100 mL of 1.0 mM FeCl3 solution containing 0.5 mol of citric acid into an equimolar K4[Fe(CN)6] solution containing 0.5 mol of citric acid under rigorous stirring at 328 K for 5 min. After acetone was added to the PB solution, the product was isolated by centrifugation for 15 min at about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and rinsed with demineralized water. The washing step was repeated four more times. Then, PB NPs solution was ready for the preparation of the PB-MCs, and the other PB NPs solution were lyophilized (219 K, 36 h) by using a freeze dryer to obtain PB NPs.11
000 rpm and rinsed with demineralized water. The washing step was repeated four more times. Then, PB NPs solution was ready for the preparation of the PB-MCs, and the other PB NPs solution were lyophilized (219 K, 36 h) by using a freeze dryer to obtain PB NPs.11
Preparation of poly(lactic acid) microcapsules
The PLA MCs was prepared by a double emulsion solvent-evaporation interfacial deposition process as previously described.19 Briefly, PLA (250 mg), (NH4)2CO3 solution (1 mL, 1%) and camphor (25 mg) were dissolved in methylene chloride (10 mL) at 293 K, using continuous probe-sonication with a certain output amplitude setting for 30 seconds. Then, the emulsion was poured into a PVA solution (5% w/v) and homogenized with a high speed homogenizer for 5 min at about 9500 rpm. Afterwards, the double W/O/W emulsion was poured into isopropanol (2% v/v in DI water) with magnetic stirring at room temperature for 3 h to make almost all the methylene chloride evaporate off and harden the capsules. Then, the microcapsules were collected by centrifugation at 5000g for 5 min and washed three times with hexane to further extract the methylene chloride from the polymer. After another washing step by DI water, some of the microcapsules were ready for the deposition of PB NPs, and the other capsules were lyophilized (219 K, 36 h) by using a freeze dryer to obtain PLA MCs.Preparation of PB-MCs
The microcapsules suspension (200 μL) was added in PEI solution (40 mL, 2 mg mL−1 in 0.5 M NaCl aqueous solution) in a 100 mL vial, shaken and mixed for about 15 min, then centrifuged at 5000g for 5 min. The supernatant was discarded and excessive non-adsorbed PEI molecules were washed by DI water for 3 times. Then, the dispersion of PB NPs was added in and the adsorption/centrifuge/wash steps were repeated to obtain the PB-MCs. The capsules were lyophilized (219 K, 36 h) by using a freeze dryer.Characterizations
The morphology and structure of the samples were tested using a scanning electron microscope (SEM) (FEI Quanta 200) and a transmission electron microscope (TEM) (JEOL, JEM-2010). The corresponding energy dispersive X-ray spectroscopy (EDS) of the samples was recorded by EDAX Genesis 2000 to verify the Fe element on microcapsules. The zeta potential of PLA MCs during the alternate deposition of PEI and PB NPs was calculated from the electrophoretic mobility measured with a Brookhaven ZetaPALS instrument. Diameter distribution and average diameter were determined by static light scattering (SLS) using a particle size distribution analyzer (Horiba LA-920). The Fourier transform infrared (FTIR) spectra were acquired with a Varian resolution Fourier transform infrared spectrometer (Varian FTS 3100, USA) in the range 4000–400 cm−1 at room temperature. In addition, both initial and final solutions (before and after the batch experiments) were analyzed by an inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500ce, Agilent Technologies, CA). The measurements were carried out in triplicates and the average values were reported.Adsorption experiment
Inactive cesium (cesium-133) was used to study adsorption behaviors. A series of batch experiments were carried out individually for cesium ions to determine the adsorption capacity of the adsorbent. The adsorption efficiency (A%) and adsorption capacity (Qe, mg g−1) were calculated from the cesium ions concentration change in the adsorption process according to the following equations:
 | (1) |
 | (2) |
Effects of environmental parameters
Results and discussion
Preparation and characterization of PB-MCs
Prussian blue immobilized poly(lactic acid) microcapsules were prepared by the electrostatic adsorption of the PB NPs on the microcapsule surfaces with the aid of polyelectrolyte.26 In order to verify this hypothesis, we performed a series of experiments. The morphology of the products was characterized by TEM and SEM measurements.27 Fig. 2a is the typical TEM image of the PB NPs. It can be seen that the product clearly shows a cubic structure. The blank PLA MCs exhibited well-defined spherical shape and smooth surface (Fig. 2b). Then, several densely distributed dark spots could be observed on the surface of PB-MCs (Fig. 2c). Compared with the PLA MCs, the PB-MCs kept their spherical shape and turned to be rough in surface after adsorption of PB NPs (Fig. 2c). And SEM showed the typical morphology of products in the SEM images (Fig. 2d–f), the Prussian blue nanoparticles were mostly found in the PLA MCs (lots of Prussian blue particles were observed on the PLA MCs surface), suggesting the successful conjugation of the PLA MCs and the PB NPs.28 The highly dispersed the PB NPs formed dense networks through their intrinsic self-assembly properties that coated the PLA MCs surfaces based on electrostatic sorption. | ||
| Fig. 2 Characterization of PB-MCs fabricated at different stages: TEM micrographs of (a) PB NPs, (b) PLA MCs and (c) PB-MCs; SEM micrographs of (d) PB NPs, (e) PLA MCs and (f) PB-MCs. | ||
The EDS spectrum of the PLA MCs, PEI-PLA MCs and the PB-MCs on the Cu plate was acquired to identify the component elements of the obtained agent.29 The blank PLA MCs exhibited the presence of C (C: 58.59%, the amount of Cu, Au was excluded) and O (O: 41.41%, the amount of Cu, Au was excluded) elements. In addition, the PEI-MCs exhibited the presence of C (C: 57.91%), O (O: 40.61%) and N (N: 1.48%, the amount of Cu, Au was excluded). Then calculation showed the presence of Fe element of 4.76% amount in total counts of the PB-MCs (C: 54.76%, N: 3.99%, O: 36.49%, the amount of Cu, Au was excluded) (Fig. 3a and S2†), further verifying the successful construction of the composite agent.
Investigation of the zeta potential is an important part of microcapsules characterization.30,31 the zeta potential of the PLA MCs, PB NPs, and PB-MCs were shown in Fig. 3b. Citric acid stabilized the PB NPs were negatively charged with zeta potential of about −15.78 ± 3.34 mV due to the presence of citric acid on the nanoparticles surface. The PLA MCs had negative surface charge of about −22.37 ± 2.24 mV. After adsorption of PEI, zeta potential of capsules was changed to 25.27 ± 3.26 mV. Then, PB NPs was deposited onto the surface of the PEI modified PLA capsule, resulting in a zeta potential of −14.03 ± 1.68 mV. After lyophilization, the encapsulated water in the inner aqueous phase of the polymeric microcapsules was sublimated, leaving the tiny cavities which could contribute to the floatation ability. The PB NPs on the outer surface can absorb the cesium ions by ion exchange mechanism, making the PB-MCs functional adsorbents for removing the cesium ions and effective separation from water.32
Prussian blue nanoparticles had a diameter of 47.89 ± 0.45 nm (Fig. S1†). The static light scattering (SLS) measurements showed the hydrodynamic diameter of the PLA MCs to be around 2.15 ± 0.02 μm (Fig. S2†). And the diameter of the PB-MCs showed 2.31 ± 0.04 μm (Fig. 3c). The SLS diameter of PLA MCs increased by ∼151 nm after electrostatic sorption. It is consistent with the TEM and SEM results. These results clearly demonstrate that the products have good dispersion without aggregation.33
To further characterize the synthesized particles, the FTIR spectra of the PLA MCs, PB NPs, and PB-MCs were taken and shown in Fig. 3d. The FTIR spectrum of PB-MCs shows typical FTIR spectrum of the CN with the peak at 2090 cm−1. The shoulder peak at 3420 cm−1 together with the small peak at 1610 cm−1 are associated with the Fe–O vibration in the surface oxidized layer of the PB-MCs.34 In the case of PB, it displays a strong characteristic peak of CN stretching vibration at 2084 cm−1. Both the PB NPs (3420 cm−1, 2090 cm−1, 1610 cm−1, 500 cm−1) and the PB-MCs (3420 cm−1, 2090 cm−1, 1610 cm−1) peaks can be clearly observed in the PB-MCs, showing that the PB NPs has been successfully immobilized on to the PLA MCs.35 According to the results of the FT-IR spectra, the PB-MCs were successfully prepared in the proposed processes. These results are in accordance with the changes of the adsorption capacities of the prepared adsorbents. However, the FTIR band of PB-MCs at 2090 cm−1 with very low intensity, it seems that the amount of deposited PB is low. Therefore, we did a experiment to measure the concentrations of PB NPs in PB-MCs by the Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). The results revealed that 5.11% of PB NPs was loaded on the surface PLA MCs. The main reasons is that the smaller surface of PLA MCs may have limited capacity to support PB NPs, because of that, it won't affect the excellent floatation ability of PB-MCs.
The evaluation of Cs sorption performance
Inactive cesium (cesium-133) was used to study adsorption behaviors. A series of batch experiments were carried out individually for cesium ions to determine the adsorption capacity of the adsorbent. To test whether cesium ions can be adsorbed on the surface of PB-MCs, the EDAX spectrum of the PB-MCs after Cs adsorption was also acquired in Fig. S4.† The results showed that Cs is being incorporated into the PLA microcapsules. In addition, the SEM micrograph of the PB-MCs after Cs adsorption indicated that the adsorbent maintain stable state.The presence of PB nanocrystal monolayer on the PB-MCs surface combined with the preferred floatation ability is expected to confer PB-MCs excellent Cs sorption performance. 30 mg PB-MCs and PLA MCs were added to 50 mL (5 mg L−1) of cesium chloride solution at pH 5.5 that were placed on a shaker at 200 rpm. The solutions were then shaken at room temperature for 12 h. After standing for 15 min, PB-MCs and PLA MCs all floated up to give a clear water (Fig. 4a and b), demonstrating excellent floatation ability, which is an important prerequisite for PB-MCs separation. This excellent floating response should be attributed to the lots of tiny cavities inside the PB-MCs. The floatation activity of this kind of structures may find wide applications as “floating separable microcapsules” in the future.
The adsorbed amount is an important variable to evaluate the Cs+ adsorption ability of the adsorbent.16 The adsorption behavior of Cs ions was investigated on the PB-MCs and PLA MCs was then measured by ICP-MS, thus giving the adsorption rate and adsorption capacity (Qe). The adsorption efficiency versus initial concentration of the Cs+ at adsorbent dosage of 30 mg with shaking time 12 h is shown in Fig. 4c. The adsorption efficiency was independent on Cs ions concentration over the whole range of the investigated Cs ions concentrations. For example, at an initial Cs ions concentration is 5 mg L−1, the adsorption efficiency was 94.82% on the PB-MCs, much higher than 10.96% on the PLA MCs. The adsorption equilibrium experiments showed that the adsorption capacity (Qe) increased with the initial concentration (C0). In addition, the samples were taken in different position to determine the cesium ions concentration, respectively. The results showed that the amount of cesium ions are consistent (Fig. S5†). Fig. 4d presented the adsorption capacity versus initial concentration of the cesium ions. The high increment and adsorption capacity indicated that the PB-MCs could remove cesium ions from the aqueous solution very effectively. For instance, the equilibrium adsorption capacity was 4.39 mg g−1 on the PB-MCs, much higher than 0.35 mg g−1 on the PLA MCs at an initial Cs ion concentration is 60 mg L−1. As can be seen in Fig. 4c and d, the adsorption capability of PB-MCs is significantly better than bulk PLA MCs. The negligible adsorption ability of PLA MCs probably resulted from the negative surface charge of about −22.37 mV, which could adsorb positive-charged ions via electrostatic interactions. The adsorption ability of PLA MCs is limited and lack of selectivity. On the contrary, the adsorption to the unmodified the PLA MCs. The concentrations of Cs ions ability of PB-MCs is the result of the ion-sieving functionality of PB NPs, which is much more powerful and selective.34 Thus, the combination of Cs adsorption ability of PB NPs and the floatation ability of PLA MCs resulted in the excellent Cs sorption performance of PB-MCs.
Effects of environmental parameters
Adsorption thermodynamics
Adsorption thermodynamics can indicate the energy effects in the adsorption process at different temperature. In this experiment, the thermodynamic nature of the sorption process were evaluated by calculating the adsorption thermodynamic parameters including Gibbs free energy (ΔG), standard enthalpy (ΔH) and standard entropy (ΔS) under the different temperatures tested. The adsorption thermodynamic parameters were calculated by the following equations.39| Kd = Qe/Ce | (3) |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (4) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd = ΔS/R − ΔH/RT Kd = ΔS/R − ΔH/RT
| (5) |
As it can be seen in Fig. 7, ΔS and ΔH can be obtained from the slope and intercept of the line from the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd and 1/T gives a linear relationship.40 Furthermore, ΔG of the process of adsorption at different temperature were listed in Table 1. The positive value of ΔH (14.4168 kJ mol−1) and the negative value of ΔG at different temperatures in the range of 4.0852–6.7083 assumed that the process of adsorption was an spontaneous and endothermic reaction.41 Moreover, the decreasing value of ΔG with increasing the temperature and the positive amount of ΔS (63.9436 J mol−1 K−1), imply that cesium adsorption on the PB-MCs is more favorable at higher temperature.
Kd and 1/T gives a linear relationship.40 Furthermore, ΔG of the process of adsorption at different temperature were listed in Table 1. The positive value of ΔH (14.4168 kJ mol−1) and the negative value of ΔG at different temperatures in the range of 4.0852–6.7083 assumed that the process of adsorption was an spontaneous and endothermic reaction.41 Moreover, the decreasing value of ΔG with increasing the temperature and the positive amount of ΔS (63.9436 J mol−1 K−1), imply that cesium adsorption on the PB-MCs is more favorable at higher temperature.
 | ||
| Fig. 7 Adsorption thermodynamics of cesium ions adsorption on PB-MCs (C0 = 5.0 mg L−1; pH = 5.5; m = 0.03 g; V = 20 mL; r = 200 rpm). | ||
| T (K) | 288 K | 298 K | 308 K | 318 K | 328 K |
| −ΔG (kJ mol−1) | 4.0852 | 4.6358 | 5.1211 | 5.8397 | 6.7083 |
Sorption isotherms
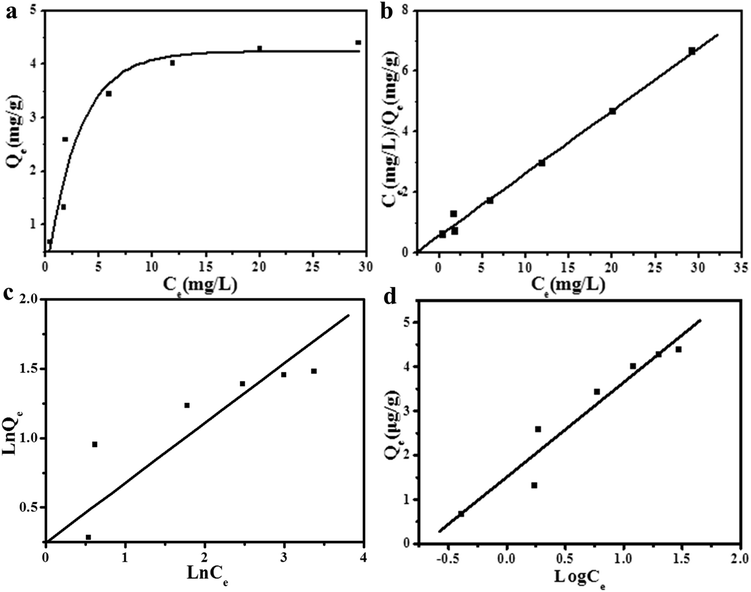
Equilibrium isotherms provide information about the sorption mechanism, surface properties, and the affinity of the sorbent. The adsorption capacity of PB-MCs adsorbent was investigated at pH 5.5 with a Cs+ solution at a certain concentration from 5 to 60 mg L−1, and shaken at 293 K for a certain time. Fig. 8a shows the adsorption isotherms of cesium ions on PB-MCs adsorbent at 293 K. In this study, the Langmuir, the Freundlich and the Temkin isotherm models were used to describe the relationship between the adsorbed amount of cesium ions and its equilibrium concentration in solution.25 The linear form of Langmuir isotherm is represented by:
 | (6) |
| RL = 1/(1 + aC0) | (7) |
The linear form of Freundlich isotherm is expressed by:
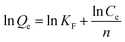 | (8) |
Qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A + B A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (9) |
The Langmuir model assumes that the uptake of the adsorbate occurs on a homogeneous surface by monolayer adsorption without any interaction between adsorbed ions due to a surface of a finite number of identical sites. The Freundlich isotherm describes the heterogeneous surface energies by multilayer adsorption. Then the Temkin isotherm can apply to the chemical adsorption, indicating that adsorption process of the adsorbate on the adsorbent was carried out by chemical adsorption.
According to the results that show in Fig. 8b–d and Table 2, higher R2 values (R2 = 0.9934) was obtained from Langmuir model for Cs+ than from the Freundlich model (R2 = 0.8670) and the Temkin model (R2 = 0.9313), and the maximum capacity of the PB-MCs is 4.8370 mg g−1, suggesting the applicability of Langmuir model to this system.43 Herein, the experimental data were reasonably fitted the Langmuir model, based on the correlation coefficient (R2).
| Sorption isotherms | Langmuir model | Freundlich model | Temkin model |
|---|---|---|---|
| 1/n | — | 0.43 | — |
| A | 4.8370 | — | 0.2445 |
| a (L mg−1) | 0.3672 | — | — |
| b (mg g−1) | — | 1.2769 | — |
| B (L g−1) | — | 0.4335 | |
| R2 | 0.9934 | 0.8670 | 0.9313 |
Moreover, the low values for parameter b (<1) and n (<3) indicated a high affinity for the PB-MCs. Furthermore, the values of RL indicates the Langmuir isotherm shapes, which can be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0).44 As shown in Table 3, the RL values were found to be in the range of 0.043–0.353 for PB-MCs, which implied that the suitability of PB-MCs as a Cs adsorbent. The results indicated that the adsorption of Cs+ by the PB-MCs was monolayer-type and adsorption process of cesium ions on the PB-MCs was carried out by chemical adsorption.
| C0 (mg L−1) | 5 | 10 | 20 | 30 | 40 | 50 | 60 |
| RL | 0.352 | 0.214 | 0.119 | 0.083 | 0.064 | 0.052 | 0.043 |
Adsorption kinetics
The necessary equilibrium time related with the contact time (t) is one of the important characteristics in order to define the efficiency of adsorption.45–47 Herein 0.03 g of the PB-MCs adsorbent was added to a 30 mL cesium ion solution (C0 (Cs+) = 40 mg L−1, pH = 5.5), and shaken at 293 K for a certain time. Fig. 9a presented the effect of contact time on the removal of cesium at 293 K. The plots indicated that Qt (mg g−1) rapidly increased with an increase of t (min) first and the adsorption process achieves almost 86% of its adsorption efficiency. Then Qt (mg g−1) reached a maximum at t (min) of 60 min and remained stable at longer times, respectively. The results indicated that the short time needed for adsorption to achieve equilibrium state could be attributed to the high adsorption efficiency and readily available adsorbing sites on the PB-MCs surface.48 The results also showed that PB-MCs can be used to remove cesium ions at shorter contacting times (60 min) rather than 12 hours. In other words, according to the requirement of cost saving and energy consumption, the PB-MCs can be applied to practical wastewater treatment applications as a commercial product shorter contacting times (60 min). Meanwhile, the pseudo-first-order, pseudo-second-order and intraparticle diffusion kinetic models were used to evaluate the sorption mechanism of PB-MCs.49The pseudo-first-order rate equation can be expressed as equation:
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − K1t Qe − K1t
| (10) |
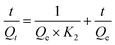 | (11) |
The intraparticle diffusion kinetic model is shown in eqn (12)
| Qt = Kit0.5 + C | (12) |
The calculated pseudo-first-order, pseudo-second-order and intraparticle diffusion models parameters are summarized in Table 4. The theoretical Qe values are the equilibrium concentrations of cesium ions in the adsorbed PB-MCs assuming 100% of Cs+ is removed. The calculated Qe values are in agreement with the theoretical ones, and the plots show quite good linearity with R2 above 0.9906. Higher R2 values (R2 = 0.9906) was obtained from the pseudo-second-order model for Cs+ than that from the pseudo-first-order kinetic model (R2 = 0.9471). Therefore, the adsorption kinetics follows the pseudo-second-order model, suggesting a chemisorption process. Meanwhile, the correlation coefficient (R2) for the pseudo second order model indicated a strong correlation, suggesting the adsorption of cesium by the described stationary phase follows the pseudo-second-order kinetic model and the rate limiting step may be chemical adsorption involving sharing or exchange of electrons, in addition adsorption follows Langmuir isotherm.13
| PB-MCs | Pseudo-first-order | Pseudo-second-order | Intraparticle diffusion (1) | Intraparticle diffusion (2) |
|---|---|---|---|---|
| K1 (min−1) | 0.0300 | — | — | — |
| K2 (g mg−1 min−1) | — | 0.0027 | — | — |
| Ki (mg g−1 min−0.5) | — | — | 0.6215 | 0.0652 |
| C | — | −0.7549 | 4.6165 | |
| Qe (mg g−1) | 5.5385 | 7.2385 | — | — |
| R2 | 0.9471 | 0.9906 | 0.9281 | 0.9960 |
In addition, the intraparticle diffusion kinetic model can be describe the adsorption process of cesium ions. Fig. 9d gives a curve included two parts, indicated that two steps in the adsorption process of cesium ions. The plots showed that cesium ions fast diffuse to the surface of the adsorbent (film diffusion) at t0.5 < 8. Meanwhile, the results suggested that cesium ions on the surface of the PB-MCs diffuse to the microporous of the adsorbent at t0.5 > 8. Meanwhile, the cure of Qt vs. t0.5 is the line disjoined to zero point from 5 min to 60 min, showed that the adsorption process of cesium ions on PB-MCs was controlled by the combination of film diffusion and intraparticle diffusion.50 On the other hand, higher Ki-1 values (Ki-1 = 0.6215) was obtained from the first stage of the straight line than that from the second stage of the straight line (Ki-2 = 0.0652) and the straight line close to the level the second stage in Fig. 9d. The data showed that the film diffusion was very fast diffuse in the first stage and the adsorption process reached adsorption equilibrium in a short time in the second stage.
Comparing the sorption capacities of other cesium adsorbents reported in the literature with the PB-MCs (Table 5), it can be seen that the PB-MCs have lower sorption capacity than PB NPs,51 but have a higher sorption capacity than latex particles functionalized with transition metals ferrocyanides and other adsorbents.55 However, this result is still promising when considering that the amount of PBNPs loaded on PLA-MCs by electrostatic adsorption was very small. In addition, the adsorption capacity has a close relationship with the characteristics of adsorbent such as the size of particle and density.55 The PB-MCs have not a higher sorption capacity than PB–Fe3O4 and PB/Fe3O4/GO NPs,38,52 but all these adsorbents have the disadvantages of involving toxic metals, while considering good dispersion, stability, no secondary environmental pollution and easy separation of PB-MCs from aqueous solutions, the PB-MCs was suitable adsorbents for the purification of radiative cesium-contaminated wastewater. The separation of PB from aqueous solutions is very difficult, which greatly restricts the application of Prussian blue as adsorbents in practical wastewater treatment applications.
Conclusions
In summary, we have first developed that an facile and effective method to combine the adsorption characteristic of PB NPs with convenient flotation separation capability of PLA MCs to synthesize Prussian blue functionalized microcapsules. The obtained PB-MCs is consisted of bubble-containing PLA MCs covered with PB nanocrystals. This adsorbent not only showed rapid flotation property due to the tiny air cavities associated with the simple flotation separation process but also exhibited a high adsorption capacity for cesium ions from aqueous solutions. By incorporating with the Cs ion-sieving functionality of PB NPs, PB-MCs showed by TEM, SEM, EDS, Zeta, FTIR, SLS, and it was successfully employed as a novel adsorbent material for separation and preconcentration of cesium ions from aqueous solution. The results showed that PB-MCs exhibited good dispersibility and stability in aqueous solutions. Meanwhile, it was found that the adsorption process can be well fitted by Langmuir isotherm model and the pseudo-second-order kinetics by the adsorption isotherms and kinetics studies, respectively. The intraparticle diffusion equation indicated that the adsorption rate of cesium on PB-MCs was controlled by the combination of film diffusion and intraparticle diffusion. In addition, it is clearly that the research highlights are combined merits of PB and PLA in this work, herein providing an effective method to prepare multi-functional composites for various applications. Most importantly, the synthesis procedure is easy and cost-effective and all materials involved are biocompatible and biodegradable. Meantime, the PB-MCs adsorbent can lower energy consumption, reduce cost and treat wastewater efficiently. The PB-MCs adsorbent has the characteristics of economy high efficiency, no secondary environmental pollution and environmental protection. This paper opens the door to bring functionalized Prussian blue microcapsules from fundamental research to practical wastewater treatment applications. Thus, the as-developed sorbents PB-MCs may pave a way to large scale treatment of radioactive cesium in environment.Acknowledgements
This research is financially supported by National Natural Science Foundation of China (No. 81371580).Notes and references
- D. Parajuli, H. Tanaka, Y. Hakuta, K. Minami, S. Fukuda, K. Umeoka, R. Kamimura, Y. Hayashi, M. Ouchi and T. Kawamoto, Environ. Sci. Technol., 2013, 47, 3800–3806 CrossRef CAS PubMed.
- M. R. Awual, S. Suzuki, T. Taguchi, H. Shiwaku, Y. Okamoto and T. Yaita, Chem. Eng. J., 2014, 242, 127–135 CrossRef CAS.
- K. Hirose, J. Environ. Radioact., 2012, 111, 13–17 CrossRef CAS PubMed.
- H. Dahlgaard, M. Eriksson, S. P. Nielsen and H. P. Joensen, Sci. Total Environ., 2004, 331, 53–67 CrossRef CAS PubMed.
- A. Yu Lonin, V. V. Levenets, I. M. Neklyudov and A. O. Shchur, J. Radioanal. Nucl. Chem., 2014, 303, 831–836 CrossRef.
- R. Chakravarty, R. Ram, K. T. Pillai, Y. Pamale, R. V. Kamat and A. Dash, J. Chromatogr. A, 2012, 1220, 82–91 CrossRef CAS PubMed.
- R. K. Gupta and S. S. Dubey, J. Polym. Res., 2005, 12, 31–35 CrossRef CAS.
- R. P. Selvam, P. K. Kulkarni and V. N. S. K. Varma, RSC Adv., 2015, 5, 69642–69650 RSC.
- K. Popa and C. C. Pavel, Desalination, 2012, 293, 78–86 CrossRef CAS.
- G. Fu, W. Liu, S. Feng and X. Yue, Chem. Commun., 2012, 48, 11567–11569 RSC.
- M. Shokouhimehr, E. S. Soehnlen, J. H. Hao, M. Griswold, C. Flask, X. D. Fan, J. P. Basilion, S. Basu and S. P. D. Huang, J. Mater. Chem., 2010, 20, 5251–5259 RSC.
- D. R. Melo, J. L. Lipsztein, C. A. de Oliveira and L. Bertelli, Health Phys., 1994, 66, 245–252 CrossRef CAS.
- A. K. Vipin, B. Y. Hu and B. Fugetsu, J. Hazard. Mater., 2013, 258, 93–101 CrossRef PubMed.
- A. Kitajima, H. Tanaka, N. Minami, K. Yoshino and T. Kawamoto, Chem. Lett., 2012, 41, 1473–1474 CrossRef CAS.
- B. Hu, B. Fugetsu, H. Yu and Y. Abe, J. Hazard. Mater., 2012, 217–218, 85–91 CrossRef CAS PubMed.
- H. J. Yang, L. Sun, J. L. Zhai, H. Y. Li, Y. Zhao and H. W. Yu, J. Mater. Chem. A, 2013, 2, 326–332 Search PubMed.
- H. Tsuji, Bio-Based Plastics: Materials and Applications, 2013, pp. 171–239 Search PubMed.
- D. Garlotta, J. Polym. Environ., 2001, 9, 63–84 CrossRef CAS.
- H. Ke, J. Wang, Z. Dai, Y. Jin, E. Qu, Z. Xing, C. Guo, X. Yue and J. Liu, Angew. Chem., Int. Ed. Engl., 2011, 50, 3017–3021 CrossRef CAS PubMed.
- L. J. Jing, X. L. Liang, X. D. Li, Y. B. Yang and Z. F. Dai, Acta Biomater., 2013, 9, 9434–9441 CrossRef CAS PubMed.
- Y. S. Jin, J. R. Wang, H. T. Ke, S. M. Wang and Z. F. Dai, Biomaterials, 2013, 34, 4794–4802 CrossRef CAS PubMed.
- B. Y. Hu, B. Fugetsu, H. W. Yu and Y. Abe, J. Hazard. Mater., 2012, 217, 85–91 CrossRef PubMed.
- M. Shokouhimehr, E. S. Soehnlen, A. Khitrin, S. Basu and S. P. D. Huang, Inorg. Chem. Commun., 2010, 13, 58–61 CrossRef CAS.
- W. W. Zhu, K. Liu, X. Q. Sun, X. Wang, Y. G. Li, L. Cheng and Z. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 11575–11582 CAS.
- M. L. Zhang, Q. F. Yao, C. Lu, Z. H. Li and W. X. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 20225–20233 CAS.
- L. Yu, Y. G. Gao, X. L. Yue, S. Q. Liu and Z. F. Dai, Langmuir, 2008, 24, 13723–13729 CrossRef CAS PubMed.
- X. Yang, Z. Dai, A. Miura and N. Tamai, Chem. Phys. Lett., 2001, 334, 257–264 CrossRef CAS.
- Z. F. Dai, L. Qun and B. X. Peng, Dyes Pigm., 1998, 36, 243–248 CrossRef CAS.
- W. Yantasee, R. D. Rutledge, W. Chouyyok, V. Sukwarotwat, G. Orr, C. L. Warner, M. G. Warner, G. E. Fryxell, R. J. Wiacek, C. Timchalk and R. S. Addleman, ACS Appl. Mater. Interfaces, 2010, 2, 2749–2758 CAS.
- X. Liang, X. Yue, Z. Dai and J. Kikuchi, Chem. Commun., 2011, 47, 4751–4753 RSC.
- X. L. Liang and X. D. Li, Angew. Chem., 2011, 123, 11826–11831 CrossRef.
- Y. Si, T. Ren, B. Ding, J. Y. Yu and G. Sun, J. Mater. Chem., 2012, 22, 4619–4622 RSC.
- S. Z. Li, X. L. Yue, Y. M. Jing, S. S. Bai and Z. F. Dai, Colloids Surf., A, 2011, 380, 229–233 CrossRef CAS.
- H. J. Yang, H. Y. Li, J. L. Zhai, L. Sun, Y. Zhao and H. W. Yu, Chem. Eng. J., 2014, 246, 10–19 CrossRef CAS.
- J. F. Zhai, Y. M. Zhai, D. Wen and S. J. Dong, Electroanalysis, 2009, 21, 2207–2212 CrossRef CAS.
- L. Kong, L. L. Yan, Z. Qu, N. Q. Yan and L. Li, J. Mater. Chem. A, 2015, 3, 15755–15763 CAS.
- M. Barkat, D. Nibou, S. Chearouche and A. Mellah, Chem. Eng. Process., 2009, 48, 38–47 CrossRef CAS.
- H. J. Yang, L. Sun, J. L. Zhai, H. Y. Li, Y. Zhao and H. W. Yu, J. Mater. Chem. A, 2014, 2, 326–332 CAS.
- X. Y. Yu, T. Luo, Y. X. Zhang, Y. Jia, B. J. Zhu, X. C. Fu, J. H. Liu and X. J. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 2585–2593 CAS.
- S. Kumar, R. R. Nair, P. B. Pillai, S. N. Gupta, M. A. R. Iyengar and A. K. Sood, ACS Appl. Mater. Interfaces, 2014, 6, 17426–17436 CAS.
- J. Q. Jiang, C. X. Yang and X. P. Yan, ACS Appl. Mater. Interfaces, 2013, 5, 9837–9842 CAS.
- M. Zhang, Q. Yao, C. Lu, Z. Li and W. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 20225–20233 CAS.
- X. D. Zhu, Y. C. Liu, C. Zhou, S. C. Zhang and J. M. Chen, ACS Sustainable Chem. Eng., 2014, 2, 969–977 CrossRef CAS.
- D. H. Ding, Z. F. Lei, Y. N. Yang, C. P. Feng and Z. Y. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 10151–10158 CAS.
- Y. Cantu, A. Remes, A. Reyna, D. Martinez, J. Villarreal, H. Ramos, S. Trevino, C. Tamez, A. Martinez, T. Eubanks and J. G. Parsons, Chem. Eng. J., 2014, 254, 374–383 CrossRef CAS PubMed.
- I. Marzouk, L. Dammak and B. Hamrouni, Water Environ. Res., 2013, 85, 99–104 CrossRef CAS PubMed.
- S. Sharma and N. C. Kothiyal, Environ. Sci. Pollut. Res., 2013, 20, 8986–8995 CrossRef CAS PubMed.
- R. Ricco, K. Konstas, M. J. Styles, J. J. Richardson, R. Babarao, K. Suzuki, P. Scopece and P. Falcaro, J. Mater. Chem. A, 2015, 3, 19822–19831 CAS.
- X. L. Yue, S. S. Feng, S. Z. Li, Y. M. Jing and C. L. Shao, Colloids Surf., A, 2012, 406, 44–51 CrossRef CAS.
- S. Wang, Y. Y. Zhai, Q. Gao, W. J. Luo, H. Xia and C. G. Zhou, J. Chem. Eng. Data, 2014, 59, 39–51 CrossRef CAS.
- C. Thammawong, P. Opaprakasit, P. Tangboriboonrat and P. Sreearunothai, J. Nanopart. Res., 2013, 15, 1689–1699 CrossRef.
- T. Sasaki and S. Tanaka, Chem. Lett., 2012, 41, 32–34 CrossRef CAS.
- S. Dahiya, R. M. Tripathi and A. G. Hegde, J. Hazard. Mater., 2008, 150, 376–386 CrossRef CAS PubMed.
- V. Avramenko, S. Bratskaya, V. Zheleznov, I. Sheveleva, O. Voitenko and V. Sergienko, J. Hazard. Mater., 2011, 186, 1343–1350 CrossRef CAS PubMed.
- X. Liu, G. R. Chen, D. J. Lee, T. Kawamoto, H. Tanaka, M. L. Chen and Y. K. Luo, Bioresour. Technol., 2014, 160, 142–149 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Size distribution PB NPs and PLA MCs (Fig. S1), the EDS spectra of PLA MCs (Fig. S2). See DOI: 10.1039/c6ra01450j |
| This journal is © The Royal Society of Chemistry 2016 |