Surface modification of banana stem fibers via radiation induced grafting of poly(methacrylic acid) as an effective cation exchanger for Hg(ii)†
Abstract
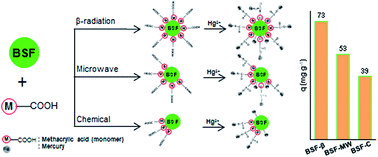
A low cost adsorbent, banana stem fibers (BSFs), was used for modification by grafting with methacrylic acid via three free radical generation methods. The presence of poly(methacrylic acid) on the adsorbent surface was verified by FTIR, ESR and TG analyses. BSFs grafted via β-radiation (BSF-β) were proven to have a higher grafting yield which led to a higher Hg(II) adsorption capacity. A slight decrease in the equilibrium pH after the adsorption process was probably due to BSF-β acting as an acid-form ion-exchanger. The adsorption equilibrium uptake fitted well with the Freundlich isotherm model implying that Hg(II) adsorption occurred heterogeneously on the adsorption sites. The kinetics of adsorption follows a pseudo-first order model with an activation energy of 13.7 kJ mol−1 indicating that the adsorption undergoes an ion-exchange process. Thermodynamic studies illustrated that that the Hg(II) adsorption process was endothermic and non-spontaneous. Spent BSF-β was effectively regenerated with 0.1 M HCl and could be reused without any significance efficiency loss over at least six cycles of adsorption. The present investigation shows that BSF-β is a promising adsorbent for the removal and recovery of Hg(II) ions from aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...