A robust and easily integrated plasma separation chip using gravitational sedimentation of blood cells filling-in high-aspect-ratio weir structure†
Abstract
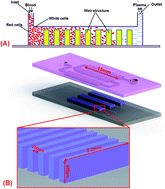
A robust and easily integrated plasma separation chip is very important for integration with microfluidic chips. Robust means that the system can tolerate small deviations in the boundary conditions (flow rate, temperature, position, hematocrit, dilutions and so on) without failure of the system. If a separation technique requires a precise flow rate and delivers other results, then the approach is not robust. Easily integrated means that the processing of fabrication and operation of the separation chip must be compatible with other parts of the microfluidic chip. In this work, a microfluidic plasma separation chip that uses the gravitational sedimentation effect to separate plasma from whole blood has been designed, fabricated and evaluated. The weir structure was constructed by using high-aspect-ratio SU-8 thick-photoresist molds, and then a double-polydimethylsiloxane (PDMS) layer was fabricated by casting, demolding and bonding with the PDMS layer. The high-aspect-ratio weir structure is beneficial for retaining blood cells in the gap of the weir structure. The conventional soft lithography process and use of PDMS materials makes it easy to integrate with other microfluidic technology. The simple weir structure is clogging-free and robust for the majority of plasma requirements. 20 μL of plasma was extracted from 65 μL of diluted blood (diluted factors ≥ 1 : 9) and the separation efficiency was above 90%.

 Please wait while we load your content...
Please wait while we load your content...