Electrically bistable and non-volatile memory devices based on p-toluenesulfonic-doped poly(triphenylamine)†
Decai Renab,
Hongling Lia,
Yu Zhua and
Xuduo Bai*a
aKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry Engineering and Materials Science, Heilongjiang University, Harbin 150080, P. R. China. E-mail: 76949956@qq.com; Tel: +86 451 86608131
bHeilongjiang East University, Harbin 150066, P. R. China
First published on 2nd March 2016
Abstract
Two novel poly(triphenylamines) (PTPAs) were prepared from 1,4-dibromobenzene, 4,4′-dibromodiphenyl ether and 4-phenoxyaniline, by a Buchwald–Hartwig coupling reaction. The structures of the resulting polymers were fully characterized by FTIR and 1H NMR. These PTPAs are thermally stable with 5% weight loss over 450 °C and the glass transition temperature (Tg) was found to be 140–149 °C. The resistive switching devices with the configuration Al/PTPA-TsOH/ITO were constructed using the conventional solution coating process. The devices demonstrated bi-directionally switchable WORM memory behavior. The ON/OFF current ratio of these devices was about 106 and the retention times can be as long as 104 s. The mechanism of the resistance switching effects of the devices can be understood on the basis of the electric field-induced doping effect, which would provide a guideline for designing new materials with high-performance memories.
Introduction
Over the years, the use of polymeric materials in optoelectronic devices has attracted significant attention, such as in transistors,1 light-emitting diodes,2 solar cells,3 and electrochromic devices4 resulting from the advantages of rich structural flexibility, low-cost, solution processability, and three-dimensional stacking capability.5 Besides these applications, polymeric memory devices6 have been investigated as a promising alternative to the traditional semiconductor-based memory devices since the first polymer electronic memory reported by Sliva et al.7 As compared to the conventional inorganic memory materials, polymeric memory materials store information in the form of ON (high current) and OFF (low current) states and have the superiority of longer data retention time, higher data storage density, fast speed, and low power consumption.8 Thus, polymeric materials with electrical bistability resulting from a conductivity difference in response to the applied electric field begin to stand out conspicuously and have predominance to face the problems and challenges in scaling down from the micro-scale to the nano-scale.The resistive-type memory devices store data based on the high (ON) and low (OFF) current state according to an applied voltage using a simple sandwich device, consisting of metal electrode/polymer/indium tin oxide (ITO) substrate, where the polymer material is used as a data storage medium. There have been a number of demonstrations for the application of polymers in memory devices. They are classified into four categories: conjugated polymers,9 polymers with pendent electro-active chromophores,10 functional polyimides,11 and hybrid composites.12
Meanwhile, acid-doped polymers have been intensively investigated and used for memory applications. Forrest spun a layer of polyethylene dioxythiophene:polystyrene sulfonic acid (PEDOT:PSS) onto the surface of a Si p–i–n thin film diode that was pre-deposited onto a stainless substrate. The device showed a WORM memory type.13 Kim attempted to dope poly(3-hexylthiophene) (P3HT) with 2-ethylbenzenesulfonic acid (EBSA) in order to improve the performance of organic field effect transistors. The doping method is effectively applied for semiconducting polymers so that it could contribute to the improved performance of organic devices such as organic memory devices.14 Recently, Li designed a very smart strategy to overcome the problem of severe fluctuations by controlling the resistance of the polymer device gradually and exactly via manipulating the doping level of a toluenesulfonic acid (TsOH):poly(schiff base) (PA) system. It was found that the Pt/PA-TsOH/Pt devices exhibited smooth, self-rectifying I–V characteristics during the resistive switching process.15
Triphenylamine (TPA) and its derivatives are well-known candidates for hole transport materials in organic photo-electronic devices due to their stable radical cations and good hole mobility.16 Besides, TPA-based polymers with good thermal stability are not only used as the hole transport layer in electroluminescent devices but also are widely applied in the fields of electrochromic17 and memory behavior.18 Recently, poly(triphenylamine) (PTPA) with electron donor and the electron acceptor moieties synthesized by an oxidative coupling reaction has been used as the functional layer in memory devices. Novel thermally stable and electron-donating PTPAs with pendant anthraquinone (AQ) electron acceptors were synthesized for investigating the side-chain and linkage-mediated effects on the memory behavior. By incorporating an isolated ether group between the TPA donor and pendant AQ acceptor, the PTPA-OAQ-based memory device revealed a longer retention time.19 Liou’s group synthesized PTPAs consisting of an intrinsic electron-donating TPA main chain and different pendant substituent acceptors. CN-PTPA exhibited a volatile DRAM memory characteristic due to a weak charge transfer capability. 2CN-PTPA and 3CN-PTPA showed a volatile SRAM memory property depending on the electron-withdrawing capability of the acceptors. Furthermore, NO2-PTPA afforded non-volatile WORM memory behavior attributed to the charge that could be trapped into the non-conjugated group.20 In contrast, PTPA as a pure electron donor system has no electrical switching capability.19,21 However, the unpaired electrons on the nitrogen in the TPA unit can act as effective atomic or molecular anchor sites for protonic acid dopants.22 Thus, TsOH-doped PTPA as a pure electron donor system was studied for memory devices in this paper. We synthesized two PTPAs: PTPA-1 and PTPA-2 (Scheme 1). The memory devices based on TsOH-doped PTPA exhibit bistable electrical switching and non-volatile memory with a high ON/OFF ratio and a long retention time.
Experimental
Materials
4-Phenoxyaniline, 1,4-dibromobenzene, p-toluenesulfonic acid, and 4,4′-dibromodiphenyl ether were purchased from TCI (Shanghai, China). Toluene was treated by the under pressure distillation method over CaH2 before use. All other reagents were used as received from commercial sources.Measurement
FTIR spectra were recorded on a Perkin Elmer Spectrum 100 Model FT-IR spectrometer. 1H NMR spectra were measured on a Bruker AC-400 MHz spectrometer using CDCl3 as solvent. Gel permeation chromatographic (GPC) analysis was performed on a Malvern instrument connected with one refractive index detector (Viscotek-VE3580-RI-DETECTOR) using a polymer/THF solution at a flow rate of 1.0 mL min−1 at 30 °C and calibrated with polystyrene standards. Thermogravimetric analysis (TGA) was conducted with approximately 6–8 mg powder samples heated in flowing nitrogen or air (gas flow rate = 40 cm3 min−1) from room temperature to 750 °C at a heating rate of 10 °C min−1 using a Perkin Elmer Pyris 6. DSC analyses were performed on a Netzsch DSC 200 F3 at a scan rate of 10 °C min−1 under a nitrogen atmosphere. CV measurements (the oxidation and reduction potentials) were conducted on a CH Instruments 660A electrochemical analyzer at a scan rate of 100 mV s−1 with a 0.1 M solution of TBAP as an electrolyte under nitrogen atmosphere in dried CH3CN. The polymer films coated on an indium tin oxide (ITO) disk, a Pt wire and a Ag/AgCl electrode were used as the working electrode, counter electrode and a quasi-reference electrode, respectively, and calibrated against the ferrocene/ferrocenium (Fc/Fc+) redox couple. UV-vis spectra were determined on a SHIMADZU UV-3600.Synthetic procedures
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– benzene skeleton stretch), 1304 (C–N stretch), 1231 (C–O stretch). 1H NMR (400 MHz in CDCl3, ppm): 6.91–7.32 (m, 13H, benzene ring).
C– benzene skeleton stretch), 1304 (C–N stretch), 1231 (C–O stretch). 1H NMR (400 MHz in CDCl3, ppm): 6.91–7.32 (m, 13H, benzene ring).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– benzene skeleton stretch), 1310 (C–N stretch), 1233 (C–O stretch). 1H NMR (400 MHz in CDCl3, ppm): 7.28–7.33 (m, 2H, Hd), 6.98–7.03 (m, 9H, Ha + Hc + He), 6.87–6.92 (m, 6H, Hb).
C– benzene skeleton stretch), 1310 (C–N stretch), 1233 (C–O stretch). 1H NMR (400 MHz in CDCl3, ppm): 7.28–7.33 (m, 2H, Hd), 6.98–7.03 (m, 9H, Ha + Hc + He), 6.87–6.92 (m, 6H, Hb).Solutions and doping process
The PTPA polymers were dissolved in chlorobenzene at a solid concentration of 10 mg mL−1. To the PTPA solutions were added TsOH molecules with a TsOH to TPA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the solutions were vigorously stirred to achieve a clear solution. These solutions were continuously stirred at 60 °C for 48 h. The process was assisted by 20 min ultrasonication before the solution was used for spin-coating.
1, and the solutions were vigorously stirred to achieve a clear solution. These solutions were continuously stirred at 60 °C for 48 h. The process was assisted by 20 min ultrasonication before the solution was used for spin-coating.
Fabrication and characterization of memory devices
The memory device was fabricated with the configuration of Al/PTPA-TsOH/ITO. The ITO glass used for the memory device was pre-cleaned by ultrasonication with water, acetone, and isopropanol each for 20 min. The solution of PTPA-TsOH was filtrated by a 0.45 μm pore size PTFE membrane syringe filter and spin-coated at 400 rpm for 12 seconds, and at 1000 rpm for 30 seconds onto the ITO substrate and kept at 80 °C overnight under nitrogen. The film thickness was determined to be around 100 nm. Finally, a 300 nm-thick Al electrode was thermally evaporated through the shadow mask (recorded device units of 0.4 × 0.4 mm2 in size) at a pressure of 10−7 torr with a uniform depositing rate of 3–5 Å s−1. The electrical characterization of the memory device was performed by a Keithley 4200-SCS semiconductor parameter analyzer equipped with a Keithely 4205-PG2 arbitrary waveform pulse generator. ITO was used as the cathode (kept constant), and Al was set as the anode during the voltage sweep.Results and discussion
Synthesis of polymers
The synthesis shown in Scheme 1 proceeded via a Pd-catalyzed polycondensation of 4-phenoxyaniline with 1,4-dibromobenzene and 4,4′-dibromodiphenyl ether. The used ligand was P(t-Bu)3, which was reported as an effective ligand in polycondensation.23 The polymers were obtained in good yields (over 70%) and were well characterized by FTIR and 1H NMR. The FTIR spectrum for PTPA-1 was used as an example to confirm polymer formation, as shown in Fig. 1, and exhibits a strong absorption peak at 3036 cm−1, which was attributed to a C–H stretch. The peaks at 1588 and 1487 cm−1 can be ascribed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– stretching vibration of the benzene ring. The absorption peak at 1304 cm−1 arises from the stretching vibration of the C–N group. The moderate peaks at 691, 748 and 830 cm−1 can be attributed to the characteristic vibration and 1,4-substitution of the benzene ring. After the reaction, the characteristic absorption of the typical amino group disappeared. The 1H NMR spectrum of the PTPA polymers shows signals with the chemical shift between 6.5 and 8.0 ppm, which belongs to the benzene ring (Fig. 2). Therefore, these characteristics prove the successful synthesis of the PTPA polymer. The 1H NMR spectrum of PTPA-2 is shown in Fig. S1.†
C– stretching vibration of the benzene ring. The absorption peak at 1304 cm−1 arises from the stretching vibration of the C–N group. The moderate peaks at 691, 748 and 830 cm−1 can be attributed to the characteristic vibration and 1,4-substitution of the benzene ring. After the reaction, the characteristic absorption of the typical amino group disappeared. The 1H NMR spectrum of the PTPA polymers shows signals with the chemical shift between 6.5 and 8.0 ppm, which belongs to the benzene ring (Fig. 2). Therefore, these characteristics prove the successful synthesis of the PTPA polymer. The 1H NMR spectrum of PTPA-2 is shown in Fig. S1.†
Molecular weight and solubility
The molecular weight and solubility behavior of PTPA was measured and is summarized in Table 1. The number-average molecular weight (Mn) of PTPA-1 and PTPA-2 is 4900 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570, with a polydispersity index of 1.52 and 1.51 by GPC analysis against a linear polystyrene standard respectively, suggesting that each PTPA was synthesized with a reasonably high molecular weight. PTPA-2 exhibited a higher molecular weight as compared with that of PTPA-1. This can be attributed to the incorporation of ether bonds in the main chain, which could reduce the rigidity and increase the reactivity of the molecular chain. Herein, the polymer prepared from 4,4′-dibromodiphenyl ether has a higher molecular weight. All polymers were very soluble in common organic solvents such as chloroform, THF and DMAc.
570, with a polydispersity index of 1.52 and 1.51 by GPC analysis against a linear polystyrene standard respectively, suggesting that each PTPA was synthesized with a reasonably high molecular weight. PTPA-2 exhibited a higher molecular weight as compared with that of PTPA-1. This can be attributed to the incorporation of ether bonds in the main chain, which could reduce the rigidity and increase the reactivity of the molecular chain. Herein, the polymer prepared from 4,4′-dibromodiphenyl ether has a higher molecular weight. All polymers were very soluble in common organic solvents such as chloroform, THF and DMAc.
| Code | Mwa | Mna | PDIa | DMAcb | THFb | CHCl3b |
|---|---|---|---|---|---|---|
| a Calibrated with polystyrene standards, using THF as the fluent at a constant flow rate of 1 mL min−1 at 30 °C. Polydispersity index (Mw/Mn).b The solubility was determined with 5 mg sample in 1 mL solvent. ++: soluble at room temperature. | ||||||
| PTPA-1 | 7470 | 4900 | 1.52 | ++ | ++ | ++ |
| PTPA-2 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
1.51 | ++ | ++ | ++ |
Thermal properties
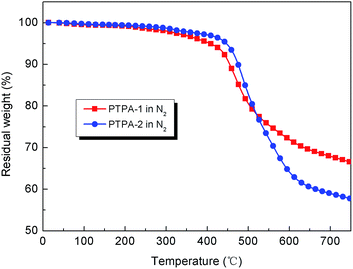
Typical TGA curves of the PTPAs in nitrogen are depicted in Fig. 3. All the polymers exhibited good thermal stability as the weight loss was less than 5% on heating to 450 °C under nitrogen atmosphere. The amount of carbonized residue (char yield) of these polymers under nitrogen atmosphere was more than 55% at 750 °C. The high char yields of the polymers can be ascribed to their high aromatic content. PTPA-1 and PTPA-2 showed a glass transition temperature (Tg) at 149 and 140 °C; PTPA-2 showed a lower Tg because of the existence of more flexible ether linkages. The thermal properties of the polymers were determined and are summarized in Table 2.| Polymer | Tga (°C) | T5db (°C) | T10db (°C) | Rw750c (%) |
|---|---|---|---|---|
| a The polymer samples were heated at 200 °C for 1 h prior to all the thermal analyses.b Temperature at which 5% and 10% weight loss occurred, respectively, recorded by TGA at a heat rate of 10 °C min−1 and gas flow rate of 40 cm3 min−1.c Residual weight percentages at 750 °C under nitrogen flow. | ||||
| PTPA-1 | 149 | 453 | 511 | 67 |
| PTPA-2 | 140 | 476 | 518 | 58 |
Electrochemical properties
Fig. 4a and b show the cyclic voltammetry (CV) of the two films. All the polymers exhibit two reversible oxidation redox couples. For PTPA-1, the first peak can be ascribed to oxidation of the electron-rich nitrogen atom in the TPA core. The second peak can be attributed to the formation of the TPA2+ dication, causing radical recombination and formation of a quinoid structure. For PTPA-2, the first peak can be ascribed to oxidation of the electron-rich nitrogen atom in the TPA unit. The second can be attributed to oxidation of phenoxy groups, causing radical recombination and formation. During the procedure of electrochemical oxidation, the color of the PTPA-1 film changed from colorless to green (L*, 70; a*, −36; b*, −8) and then blue (L*, 24; a*, 4; b*, −38), and for PTPA-2 the color changed from colorless to blue (L*, 61; a*, −43; b*, −35) and then red (L*, 41; a*, 40, b*, 38). Meanwhile PTPA-1 exhibited a lower oxidation potential of 0.75 V than that of PTPA-2 of 0.97 V, attributed to conjugation structures in the main chain. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of the two films could be calculated from the optical band gap (Eg) and the onset oxidation potential (Eonsetox) based on the reference energy level of the ferrocene/ferrocenium couple (EFOC) by the following equations respectively: EHOMO = −[Eonsetox + 4.8 − EFOC], and ELUMO = EHOMO − Eg. The optical band gap (Eg) of PTPA from the UV-vis absorption edges is estimated to be 3.05 and 3.48 eV, respectively. The HOMO energy levels of the two films were determined to be −4.85 and −5.14 eV, and the LUMO energy levels of the two films were determined to be −1.80 and −1.66 eV (Table 3), respectively. The energy barriers between the work function (Φ) of the ITO bottom electrodes (−4.8 eV) and the HOMO energy levels of PTPA-1 and PTPA-2 were 0.05 and 0.34 eV, respectively, which were significantly lower than the energy barriers of 2.48 and 2.62 eV between the work function (Φ) of the Al top electrodes (4.28 eV) and LUMO energy levels of the two films. The lower energy barriers were expected to be beneficial for enhancing hole injection from ITO to the two electro-active thin films, which indicated that holes predominated the conduction process in these devices. Hence, the two molecules exhibited p-type semiconductor characteristics.24 Fig. S4† shows the CV diagrams of the PTPA-1-TsOH and PTPA-2-TsOH film in 0.1 M TBAP acetonitrile. These films exhibited oxidation potentials of 0.93 and 1.12 V, respectively. Compared to pristine PTPA, the oxidation potentials of PTPA-TsOH are higher, respectively. It can be attributed to the formation of proton-doped TPA groups. The oxidation peak of the TPA groups, which is affected by the negatively-charged counter ions (TsO−), shifts positively. Only one reversible oxidation redox couple existed because further oxidation can be difficult in the PTPA-TsOH system. | ||
| Fig. 4 Cyclic voltammetric diagrams of the cast films of PTPA: (a) PTPA-1 and (b) PTPA-2 on ITO-coated glass substrate in 0.1 M TBAP acetonitrile. | ||
| Code | λmax (nm) | λonset (nm) | Eonseta (eV) | Egb (eV) | HOMOc (eV) | LUMOc (eV) |
|---|---|---|---|---|---|---|
| a From cyclic votammograms versus Ag/AgCl in CH3CN.b Band gaps calculated from absorption edge of the polymer films. Eg = 1240/λonset.c The HOMO energy levels were calculated from cyclic voltammetry and were referenced to ferrocene. LUMO = HOMO − Eg. | ||||||
| PTPA-1 | 335 | 407 | 0.49 | 3.05 | −4.85 | −1.80 |
| PTPA-2 | 316 | 356 | 0.78 | 3.48 | −5.14 | −1.66 |
Memory device characteristics
The memory behaviors were investigated based on current–voltage (I–V) characteristics of an Al/PTPA-TsOH/ITO sandwich device as depicted in Fig. 5. Within the sandwich device, a TsOH-doped polymer film was used as an active layer between Al and ITO as the top and bottom electrodes. To exclude the effect of polymer film thickness on the memory properties, all polymer films were cast from chlorobenzene solutions with the concentration of 10 mg mL−1. The thickness of the films was round 100 nm as shown in Fig. 5, which was measured by SEM.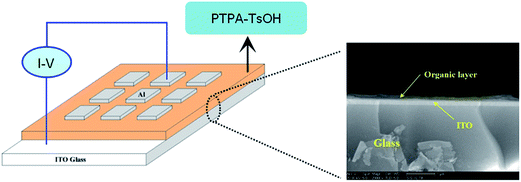 | ||
| Fig. 5 Schematic diagram of the sandwich device (left); SEM image of a cross-section view of the device (right). | ||
Fig. S5(a) and (b)† demonstrate the I–V results of pristine PTPA-1 and PTPA-2. Both the memory devices of pristine PTPA-1 and PTPA-2 were kept at a low conductivity (OFF) state during the positive and negative scans, and there was no electrical switching capability indicating an insulator characteristic. The current–voltage (I–V) characteristics of the Al/PTPA-TsOH/ITO device are shown in Fig. 6. The devices based on TsOH-doped PTPA-1 and PTPA-2 exhibit similar behavior. Herein, we take PTPA-1 (Fig. 6a) as an example to describe the bistable behavior of the memory device. In the first voltage scan from 0 to −6.0 V, the devices are typically in a low resistance state at voltages below −5.1 V. This state could be considered as the ON state of the memory. On applying a relatively high voltage, more than −5.1 V, an abrupt decrease in the current was observed for the device, which becomes the OFF state of the memory. This electrical transition represents the writing process for the memory device. The devices were kept in the OFF state for all subsequent sweeps. Thus, the devices were used as write-once-read-many times (WORM) devices. It is noteworthy that when the top electrode was initially positive biased, the device also showed a WORM memory effect. This indicates that the devices have bi-directionally switchable characteristics. The devices are considered set and can no longer be rewritten, edited, or tampered with, emphasizing their potential use in security and data protection applications.
The stability of the device under a constant stress of −2.0 V is shown in Fig. 7. An ON/OFF current ratio of about 106 can be maintained, and no degradation in the current for the ON and OFF states was observed during the test. An ON/OFF current ratio of more than 103 is achieved for this memory device. This respectable ON/OFF current ratio indicates a distinct difference between the ON and OFF state, thus promising a low misreading rate of the memory device in actual device applications.
 | ||
| Fig. 7 Retention characteristics of both the ON and OFF state for the Al/PTPA-TsOH/ITO device with a constant stress (−2 V) at room temperature. | ||
To investigate the electrical switching characteristics of our devices, the measured I–V data were further analyzed in detail using various conduction models.25 The trap-limited space-charge limited conduction (SCLC) model was found to satisfactorily fit the I–V data for the OFF states (Fig. 8). Taking PTPA-1 as an example, the logarithmic plot of the I–V data for the OFF state contains two linear regions for <1.1 and >1.1 V, with the slopes of 1.48 and 2.32, respectively. These results indicate that the trap-limited SCLC mechanism is dominant in the OFF states of the devices. The ohmic contact model was found to fit the I–V data well for the OFF states at the ON state, indicating that ohmic conduction is dominant in the corresponding states of the devices.
 | ||
| Fig. 8 Log–log plot of the I–V curves measured for the Al/PTPA-TsOH/ITO device: (a) PTPA-1, and (b) PTPA-2. | ||
Switching mechanism
The mechanism of the resistance switching effects of the devices can be understood on the basis of the electric field-induced doping/de-doping effect. Molecular doping of PTPA with a protonic acid leads to an increased concentration of the effective charge carriers in the bulk heterojunction blend of the polymer hosts and small molecule dopants. During the doping process in solution, a partial doping level is achieved in the PTPA-TsOH thin film. While under external electric fields, electromigration of the TsOH dopants toward the nitrogen atoms gives rise to an enhanced doping level of PTPA.13a,15,26 As a consequence, the increased concentration of charge carriers leads to a consecutive increase in the device conductivity. The hole transporting energy bands (paths) are created by both field effect and TsOH doping. Thus, the devices are in the ON state under a low current. When the voltage bias exceeds a certain value, the large current may cause a doping structure change and rupture of the conduction channels formed in the switch OFF process. We do not observe any reversible behavior; this indicates that the doping process is difficult to reform or the rate is considerably less than their rupture. The stable WORM behavior of our devices is further confirmed.Conclusions
Two novel poly(triphenylamines) (PTPAs) were prepared from 1,4-dibromobenzene, 4,4′-dibromodiphenyl ether and 4-phenoxyaniline, by the Buchwald–Hartwig coupling reaction. The memory device with the configuration of Al/PTPA-TsOH/ITO exhibited bistable electrical switching and non-volatile WORM memory storage performance because of the electric field-induced doping/de-doping effect. The devices will be considered set and can no longer be rewritten, edited or tampered, so could be used as promising candidates in security and data protection applications. The devices revealed excellent retention of both the OFF and ON states and an ON/OFF current ratio as high as 106 in both positive and negative voltage sweeps. The proposed structural design concept of the polymers to modulate the memory behavior can be envisaged as a suitable approach towards future active memory elements in data storage applications.Acknowledgements
The authors are grateful to the support of the National Science Foundation of China (Grant no. 21372067, 51373049), Doctoral Fund of Ministry of Education of China (20132301110001 and 20132301120004), and Foundation of Heilongjiang Education Bureau (Grant No. 12543046).Notes and references
- (a) H. Yan, Z. H. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dotz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679–U1 CrossRef CAS PubMed; (b) Y. H. Chou, H. J. Yen, C. L. Tsai, W. Y. Lee, G. S. Liou and W. C. Chen, J. Mater. Chem. C, 2013, 1, 3235–3243 RSC.
- (a) R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Logdlund and W. R. Salaneck, Nature, 1999, 397, 121–128 CrossRef CAS; (b) Y. Shao, X. Gong, A. J. Heeger, M. Liu and A. K. Y. Jen, Adv. Mater., 2009, 21, 1972–1975 CrossRef CAS.
- (a) G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CAS; (b) C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15–26 CrossRef CAS; (c) M. H. Chen, J. Hou, Z. Hong, G. Yang, S. Sista, L. M. Chen and Y. Yang, Adv. Mater., 2009, 21, 4238–4242 CrossRef CAS.
- (a) H. J. Yen and G. S. Liou, Polym. Chem., 2012, 3, 255–264 RSC; (b) E. Puodziukynaite, J. L. Oberst, A. L. Dyer and J. R. Reynolds, J. Am. Chem. Soc., 2012, 134, 968–978 CrossRef CAS PubMed; (c) E. P. Knott, M. R. Craig, D. Y. Liu, J. E. Babiarz, A. L. Dyer and J. R. Reynolds, J. Mater. Chem., 2012, 22, 4953–4962 RSC.
- A. Stikeman, Technol. Rev., 2002, 105, 31 Search PubMed.
- (a) Q. D. Ling, F. C. Chang, Y. Song, C. X. Zhu, D. J. Liaw, D. S. H. Chan, E. T. Kang and K. G. Neoh, J. Am. Chem. Soc., 2006, 126, 8732–8733 CrossRef PubMed; (b) Y. Q. Li, Y. Y. Chu, R. C. Fang, S. J. Ding, Y. L. Wang, Y. Z. Shen and A. M. Zheng, Polymer, 2012, 53, 229–240 CrossRef CAS; (c) T. Kurosawa, T. Higashihara and M. Ueda, Polym. Chem., 2013, 4, 16–30 RSC.
- P. O. Sliva, G. Dir and C. Griffiths, J. Non-Cryst. Solids, 1970, 2, 316–320 CrossRef CAS.
- (a) X. D. Zhuang, Y. Chen, G. Liu, P. P. Li, C. X. Zhu, E. T. Kang, K. G. Noeh, B. Zhang, J. H. Zhu and Y. X. Li, Adv. Mater., 2010, 22, 1731–1735 CrossRef CAS PubMed; (b) F. L. Ye, P. Y. Gu, F. Zhou, H. F. Liu, X. P. Xu, H. Li, Q. F. Xu and J. Lu, Polymer, 2013, 54, 3324–3333 CrossRef CAS.
- (a) Q. D. Ling, Y. Song, S. L. Lim, E. Y. H. Teo, Y. P. Tan, C. Zhu, D. S. H. Chan, D. L. Kwong, E. T. Kang and K. G. Neoh, Angew. Chem., Int. Ed., 2006, 45, 2947–2951 CrossRef CAS PubMed; (b) X. D. Zhuang, Y. Chen, B. X. Li, D. G. Ma, B. Zhang and Y. Li, Chem. Mater., 2010, 22, 4455–4461 CrossRef CAS; (c) Y. K. Fang, C. L. Liu, C. Li, C. J. Lin, R. Mezzenga and W. C. Chen, Adv. Funct. Mater., 2010, 20, 3012–3024 CrossRef CAS.
- (a) S. J. Liu, P. Wang, Q. Zhao, H. Y. Yang, J. Wong, H. B. Sun, X. C. Dong, W. P. Lin and W. Huang, Adv. Mater., 2012, 24, 2901–2905 CrossRef CAS PubMed; (b) S. G. Hahm, N. G. Kang, W. Kwon, K. Kim, Y. K. Ko, S. Ahn, B. G. Kang, T. Chang, J. S. Lee and M. Ree, Adv. Mater., 2012, 24, 1062–1066 CrossRef CAS PubMed.
- (a) Q. D. Ling, F. C. Chang, Y. Song, C. X. Zhu, D. J. Liaw, D. S. H. Chan, E. T. Kang and K. G. Neoh, J. Am. Chem. Soc., 2006, 128, 8732–8733 CrossRef CAS PubMed; (b) K. Kim, H. J. Yen, Y. G. Ko, C. W. Chang, W. Kwon, G. S. Liou and M. Ree, Polymer, 2012, 53, 4135–4144 CrossRef CAS; (c) T. Kurosawa, Y. C. Lai, T. Higashihara, M. Ueda, C. L. Liu and W. C. Chen, Macromolecules, 2012, 45, 4556–4563 CrossRef CAS.
- (a) T. W. Kim, D. F. Zeigler, O. Acton, H. L. Yip, H. Ma and A. K. Y. Jen, Adv. Mater., 2012, 24, 828–833 CrossRef CAS PubMed; (b) M. H. Lee, J. H. Jung, J. H. Shim and T. W. Kim, Org. Electron., 2011, 12, 1341–1345 CrossRef CAS.
- (a) S. Moller, C. Perlov, W. Jackson, C. Taussig and S. R. Forrest, Nature, 2003, 426, 166–169 CrossRef PubMed; (b) X. Xu, R. A. Register and S. R. Forrest, Appl. Phys. Lett., 2006, 89, 142109–142111 CrossRef.
- S. Nam, J. Kim, H. Lee, H. Kim, C. S. Ha and Y. Kim, ACS Appl. Mater. Interfaces, 2012, 4, 1281–1288 CAS.
- B. L. Hu, X. J. Zhu, X. X. Chen, L. Pan, S. S. Peng, Y. Z. Wu, J. Shang, G. Liu, Q. Yan and R. W. Li, J. Am. Chem. Soc., 2012, 134, 17408–17411 CrossRef CAS PubMed.
- (a) Y. Shirota, J. Mater. Chem., 2005, 15, 75 RSC; (b) K. Y. Chiu, T. H. Su, C. W. Huang, G. S. Liou and S. H. Cheng, J. Electroanal. Chem., 2005, 578, 283–287 CrossRef CAS.
- (a) H. J. Yen and G. S. Liou, Chem. Mater., 2009, 21, 4062–4070 CrossRef CAS; (b) H. J. Yen and G. S. Liou, Polym. Chem., 2012, 3, 255–264 RSC.
- (a) J. C. Hsu, Y. Chen, T. Kakuchi and W. C. Chen, Macromolecules, 2011, 44, 5169–5177 CrossRef; (b) Y. C. Hu, C. J. Chen, H. J. Yen, K. Y. Lin, J. M. Yeh, W. C. Chen and G. S. Liou, J. Mater. Chem., 2012, 22, 20394–20402 RSC; (c) C. W. Chang, G. S. Liou and S. H. Hsiao, J. Mater. Chem., 2007, 17, 1007–1015 RSC.
- J. H. Wu, H. J. Yen, C. Y. Hu and G. S. Liou, Chem. Commun., 2014, 50, 4915–4917 RSC.
- J. H. Wu and G. S. Liou, ACS Appl. Mater. Interfaces, 2015, 7, 15988–15994 CAS.
- C. J. Chen, Y. C. Hu and G. S. Liou, Chem. Commun., 2013, 49, 2804–2806 RSC.
- Y. Q. Wang, Y. Liang, J. Y. Zhu, X. D. Bai, X. K. Jiang, Q. Zhang and H. J. Niu, RSC Adv., 2015, 5, 11071–11076 RSC.
- (a) T. Yamamoto, M. Nishiyama and Y. Koie, Tetrahedron Lett., 1998, 39, 2367–2370 CrossRef CAS; (b) T. Kanbara, M. Oshima, T. Imaysu and K. Hasegawa, Macromolecules, 1998, 31, 8725–8730 CrossRef CAS; (c) B. J. Jung, J. I. Lee, H. Y. Chu, L. M. Do and H. K. Shim, Macromolecules, 2002, 35, 2282–2287 CrossRef CAS.
- Y. Li, R. Fang, S. Ding and Y. Shen, Macromol. Chem. Phys., 2011, 212, 2360–2370 CrossRef CAS.
- Y. Q. Li, Y. Y. Chu, R. C. Fang, S. J. Ding, Y. L. Wang, Y. Z. Shen and A. Zheng, Polymer, 2012, 53, 229–240 CrossRef CAS.
- R. Sim, M. Y. Chan, A. S. W. Wong and P. S. Lee, Org. Electron., 2011, 12, 185–189 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01410k |
| This journal is © The Royal Society of Chemistry 2016 |