Isatin thiosemicarbazone-blended polymer films for biomedical applications: surface morphology, characterisation and preliminary biological assessment
Abstract
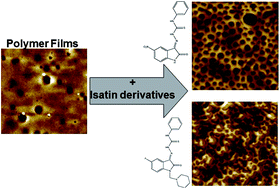
Poly(methyl methacrylate) and polyurethane are polymers currently used for a range of biomedical applications. To modify their surface characteristics, biocompatibility, and potentially reduce any related side effects, the addition to the polymers of appropriate compounds has been investigated. Isatin thiosemicarbazone derivatives were synthesised and added to poly(methyl methacrylate) and polyurethane solutions before spin-coating them on to a silica wafer. The resultant films were characterised with contact angle goniometry and atomic force microscopy. PMMA films produced from tetrahydrofuran solvent displayed honeycombed structures that were highly hydrophobic; however, such changes were not seen for polyurethane surfaces. The cytotoxicity and effect on cell proliferation of polymer surfaces were investigated using PNT2A prostate cells. The isatin-containing polymers were deemed non-toxic at the concentrations used, while cell proliferation studies suggested that the resulting films were supportive of cell growth.


 Please wait while we load your content...
Please wait while we load your content...