Novel bismuth compounds: synthesis, characterization and biological activity against human adenocarcinoma cells†
M. Ardaa,
I. I. Ozturk*a,
C. N. Banti*b,
N. Kourkoumelis*c,
M. Manolid,
A. J. Tasiopoulosd and
S. K. Hadjikakou*b
aDepartment of Chemistry, Namık Kemal University, 59030, Tekirdag, Turkey. E-mail: iiozturk@nku.edu.tr
bSection of Inorganic and Analytical Chemistry, Department of Chemistry, University of Ioannina, 45110 Ioannina, Greece. E-mail: cbanti@cc.uoi.gr; shadjika@uoi.gr; Fax: +30-26510-08786; Tel: +30-26510-08374
cMedical Physics Laboratory, Medical School, University of Ioannina, Ioannina, 45110, Greece. E-mail: nkourkou@uoi.gr
dDepartment of Chemistry, University of Cyprus, Nicosia, Cyprus
First published on 15th March 2016
Abstract
Five new bismuth(III) halide compounds (BiX3, X = Br or I) of formulae {[BiBr(Me2DTC)2]n} (1), {[BiBr2(Et2DTC)]n} (2), {[BiI2(Me2DTC)]n} (3), {[BiI(Et2DTC)2]n} (4) and {[BiI(μ2-I)(Et2DTC)2]2}n (5) (Me2DTCH = dimethyldithiocarbamate, C3H7NS2 and Et2DTCH = diethyldithiocarbamate, C5H11NS2) were synthesized from the reactions between bismuth(III) bromide (BiBr3) or bismuth(III) iodide (BiI3) with tetramethylthiuram monosulfide (Me4tms), tetramethylthiuram disulfide (Me4tds) or tetraethylthiuram disulfide (Et4tds). The complexes were characterized by melting point, elemental analysis, FT-IR spectroscopy, Raman spectroscopy, 1H, 13C NMR spectroscopy and Thermal Gravimetry-Differential Thermal Analysis (TG-DTA). Moreover, the crystal structures of 1–5 were determined with single crystal X-ray diffraction analysis. The ligands of compounds 1–5 were derived from reduction with concomitant degradation to dithiocarbamates. Complexes 1–4 are polymers, whereas complex 5 is a dimer, built up from monomeric units with square pyramidal (SP) geometry (1, 4 and 5) and pentagonal bipyramidal geometry (2 and 3) around the bismuth center. Complexes 1–5 were evaluated for their in vitro cytotoxic activity against human adenocarcinoma breast (MCF-7) and cervix (HeLa) cells. The toxicity on normal human fetal lung fibroblast cells (MRC-5) is also evaluated. Since estrogen receptors (ERs) are located in MCF-7, in contrast to HeLa cells, the estrogenic effect of 1–5 on MCF-7 cells was studied by means of a methylene blue assay. Hirshfeld surface volumes were analyzed to clarify the nature of the intermolecular interactions. Molecules with lower H-all atoms inter-molecular interactions tend to exhibit higher activity against both MCF-7 and HeLa cells. Structure–activity relationship (SAR) studies were performed for these complexes using 2D topological based disparity analysis. This finding underlines the significance of the halogen atoms in the coordination sphere of the metal ion.
1. Introduction
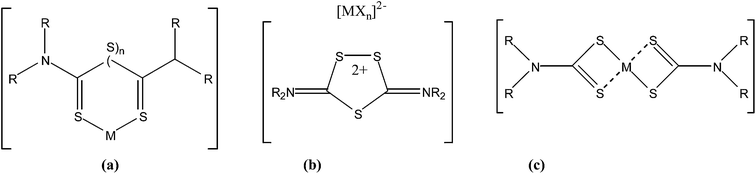
Bismuth is the heaviest stable element in the periodic table, and is recognized as a low-toxicity metal.1,2 Bismuth compounds have been widely used in medicine for more than 200 years and new bismuth-containing drugs are now being developed.3 Currently, the major medicinal use of bismuth compounds is focused in two fields: antimicrobial and anticancer.4 In the antimicrobial field, bismuth compounds (bismuth subcitrate (De-Nol®), bismuth subsalicylate (Pepto-Bismol®), ranitidine bismuth citrate (Tritec® and Pylorid®)) have been used in the treatment of various microbial infections.5 Anticancer activities of a series of bismuth tropolones complexes were examined recently.6 Early studies showed that bismuth compounds exhibit anticancer activities such as {[Bi(tgn)3(H2O)]·3.5H2O} (tgn: thioguanine) and {Na2[BiO(mp)3]·H2O} (mp: 6-mercaptopurine).7,8 Moreover, new studies have shown that in terms of developing new drug candidates based on bismuth, bismuth thiolates or sulfur containing ligands are very useful.9 Thus, bismuth xanthate complexes (Bi(S2COR)3) and bismuth dithiocarbamates complexes (Bi(S2CNR2)3) exhibit potent in vitro cytotoxic activity against cancer cell lines.10,11 Bi(III) of thiosemicarbazones complexes were recently studied for their antiproliferative activity against human leukaemia (K562), human colorectal (HCT-116), HeLa and human hepatocellular carcinoma (HepG2) cells.10d–fThe general formula of the thiuram sulfide derivatives is R2NC(S)SnC(S)NR2.12 Thiuram disulfides (R4tds) have been used as fungicides, as therapy against alcoholism and as arrestors of human immunodeficiency virus infections such as AIDS. While, thiuram monosulfides inhibit peptidyl-prolyl cis/trans isomerase activity, in HeLa cells.13 The reaction chemistry of thiuram mono- and disulfides lead to three different categories of products: (a) adducts; (b) thiuram oxidation products and (c) ligand reduction with concomitant degradation to dithiocarbamate and/or thiocarboxamide ligands (Fig. 1).14
Examples of thiuram monosulfides or disulfides adducts (Fig. 1a), include the complexes: [Zn(Me4tms)I2] (Me4tms: tetramethylthiuram monosulfide),15a, [Hg(Et4tds)I2] (Et4tds: tetraethylthiuram disulfide),15b [CuCl(Me4tms)]2, [CuBr(Me4tms)]n, [CuI(Me4tms)]2, [CuCl(Et4tms)] (Et4tms: tetraethylthiuram monosulfide).15c Besides, five membered dicationic cyclic derivatives are neutralized by metal halides counter anions (Fig. 1b) may obtained; e.g. [Et4biit-3]2+[Hg2I6]2− (Et4biit-3: 3,5-bis(N,N′diethylimonium)-1,2,4-trithiolane),16a [Et4biit-3]2+2[FeCl4]− and [Bu4biit-3]2+[Cu2X6]2− (Bu4biit-3: 3,5-bis(N,N′dibutylimonium)-1,2,4-trithiolane, X: Cl, Br).16b In the case of ligands degradation (Fig. 1c), the S–S bond is cleaved resulting in the formation of dithiocarbamate and/or thiocarboxamide fragments. These fragments can coordinate to metal ions. Examples of ligand reduction with simultaneous ligand degradation include: Tl(Me2dtc)3,17a [V2(μ-S2)2(Et2dtc)4],17b [Mo(R2dtc)4] (R: Me, Et, Ph),17c–e [Cu(Et2dtc)]4, [Cu{(i-Pr)2dtc}Br2],15c [Me3Sb(dtc)2],17f {[SbCl(Me2dtc)2]n}, {[BiCl(Me2dtc)2]n}, {[Bi(Et2dtc)3]2}.11
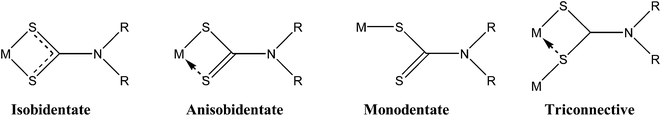
Dithiocarbamates already play an important role in medicine, as antidotes in heavy-metal detoxification.10b Dithiocarbamates exhibit strong tendency for metal ions complexation with a variety of coordination modes (Fig. 2), especially with antimony(III) and bismuth(III).17f,11,18–20 Metal–dithiocarbamate complexes have also been investigated for their anti-cancer potential, most notably with platinum(IV) palladium(II), tin(IV) and gold(I/III).10b
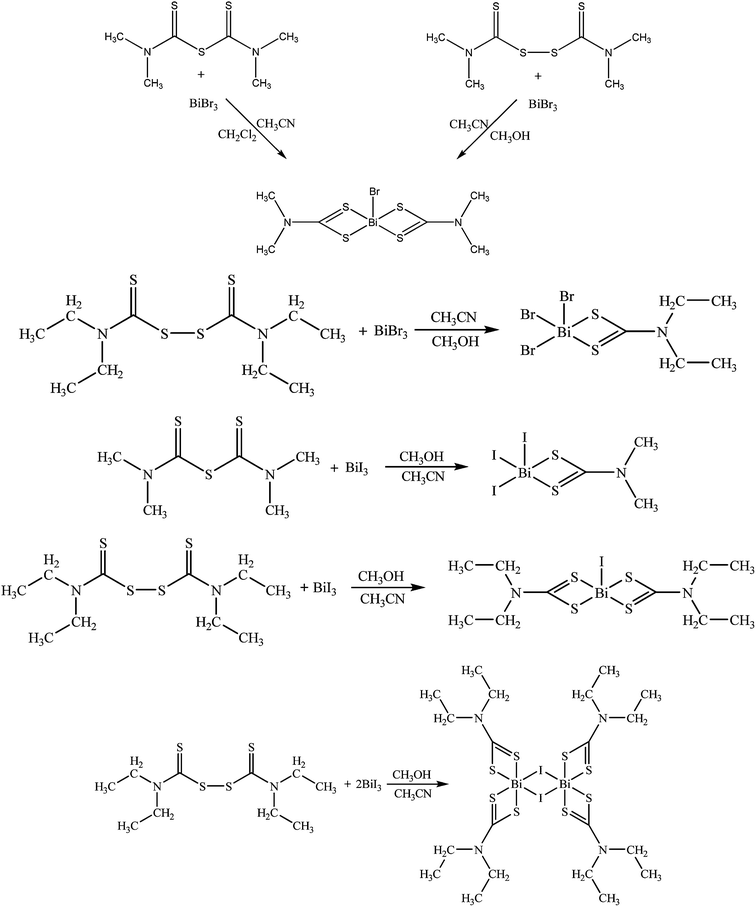
In the progress of our studies on the synthesis, characterization and study of biological activity of complexes containing metal ions of 15 group,11,21 we have synthesized and characterized five new bismuth(III) bromide and bismuth(III) iodide complexes using the tetramethylthiuram monosulfide (Me4tms), tetramethylthiuram disulfide (Me4tds) and tetraethylthiuram disulfide (Et4tds) ligands. The bismuth(III) halide complexes derived by degradation of thiuram mono- or di-sulfides are of formulae {[BiBr(Me2DTC)2]n} (1), {[BiBr2(Et2DTC)]n} (2), {[BiI2(Me2DTC)]n} (3), {[BiI(Et2DTC)2]n} (4) and {[BiI(μ2-I)(Et2DTC)2]2}n (5). Compounds 1–5 have been characterized by a variety of analytical methods; FT-IR, FT-Raman, 1H, 13C NMR, TGA-DTA and single crystal X-ray diffraction (XRD) analysis. Compounds 1–5 were also in vitro tested for their cytotoxicity against human adenocarcinoma breast (MCF-7), cervix (HeLa) and normal human fetal lung fibroblast (MRC-5) cell lines. Hirshfeld surface volumes were also determined. Structure–activity relationship (SAR) studies were performed for this complexes using 2D topological based disparity analysis.
2. Results and discussion
2.1. General aspects
Complex 1 was derived by two different routes (1a and 1b) (Scheme 1): by reacting tetramethylthiuram monosulfide with bismuth(III) bromide in acetonitrile/dichloromethane solutions (1a) or by reacting tetramethylthiuram disulfide with bismuth(III) bromide in acetonitrile/methanol solutions (1b) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to metal ratio (Scheme 1).
1 ligand to metal ratio (Scheme 1).
Complexes 2–5 have been synthesized by reacting the appropriate thiuram sulfides with an excess of bismuth(III) halides (X: Br or I) in acetonitrile/methanol solutions, as shown by the following equations (Scheme 1). During this reaction the S–S or C–S bonds are clipped and dithiocarbamate and/or thiocarboximade fragments formed coordinate to the metal ions.
2.2. Vibrational spectroscopy
Vibrational spectroscopic data were obtained for the five bismuth(III) complexes 1–5 and their thiuram ligands for comparative purposes, are summarized in Table 1 (Fig. S1–S8†). The characteristic vibrational bands that are sensitive to molecular structure are the stretching modes for the ν(CN), ν(CS), ν(BiS) and ν(BiX) bands (X = Br or I).11 The IR spectra of bismuth(III) complexes show distinct vibrational bands at 1514 (1), 1525 (2), 1520 (3), 1502–1483 (4) and 1497 (5) cm−1 respectively, which are assigned to the ν(CN) vibrations and at 964–831 cm−1 (1), 976–839 cm−1 (2), 955–841 cm−1 (3), 984–837 cm−1 (4) and 978–839 cm−1 (5) respectively, which are attributed to the ν(CS) vibrations. The IR spectra of 1–5 show two ν(CS) vibration bands and one strong ν(CN) band. This is an indication of the anisobidentate character of the S2CNR2 ligands.22 The corresponding ν(CN) and ν(CS) vibration bands of the free ligands are found at 1514–1497, 957 and 858 cm−1 for Me4tms, 1495, 968 and 847 cm−1 for Me4tds and 1496, 958 and 858 cm−1 for Et4tds, respectively.23| Mid-IR | Raman | ||||
|---|---|---|---|---|---|
| ν(CN) | ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) S) |
ν(C–S) | ν(Bi–X) | ν(Bi–S) | |
| Me4tms | 1514–1497s | 957s | 858m | — | — |
| Me4tds | 1495s | 968s | 847m | — | — |
| Et4tds | 1496s | 958s | 858m | — | — |
| {[BiBr(Me2DTC)2]}n (1) | 1514s | 964s | 831w | 172 | 371 |
| {[BiBr2(Et2DTC)]}n (2) | 1525s | 976m | 839m | 175 | 379 |
| {[BiI2(Me2DTC)]}n (3) | 1520s | 955m | 841m | 162 | 372 |
| {[BiI(Et2DTC)2]}n (4) | 1502–1483s | 984m | 837m | 164 | 362–371 |
| {[BiI(μ2-I)(Et2DTC)2]2}n (5) | 1497s | 978m | 839m | 167 | 372 |
Raman spectra of complexes 1 and 2 (Figure S9–S10†) show distinct vibrations bands at 172 and 175 cm−1 which are assigned to ν(Bi–Br) vibrations.24 The bands at 162, 164 and 167 cm−1 in the Raman spectra of 3, 4 and 5 (Fig. S11–S13†) are due to the ν(Bi–I) vibrations, respectively.24 The Raman spectra of 1–5 show distinct vibration bands at 371 (1), 379 (2), 372 (3), 362–371 (4) and 372 (5) cm−1 respectively, which are attributed to the ν(Bi–S) vibration bands.24
2.3. Thermal analysis
The thermal stability of the bismuth(III) complexes was checked through TG-DTA analysis. TG-DTA diagrams of bismuth(III) complexes 1–5, (under N2) were recorded in the temperature range 30–750 °C (Fig. S14–S18†). Complexes are stable up to 138 (1), 223 (2), 203 (3), 226 (4) and 218 (5) °C. The thermal analysis of 1–5 show one decomposition step 138–397 °C (1), 223–401 °C (2), 203–402 °C (3), 226–408 °C (4) and 218–393 °C (5) involve 99.3% (1), 88.8% (2), 96.8% (3), 79.0% (4) and 90.5% (5) mass losses.2.4. NMR spectroscopy
1H and 13C-NMR chemical shifts of the free ligands and those of the 1–5 in DMSO-d6 are summarized in Table 2 (Fig. S19–S26†).| Compounds | 1H NMR chemical shift (δ ppm) | 13C NMR chemical shift (δ ppm) |
|---|---|---|
| Me4tms | 3.38–3.42, d, 12H, (CH3– of Me4tms) | 43.79 (CH3– of Me4tms), 44.48 (CH3– of Me4tms), 186.12 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S of Me4tms) S of Me4tms) |
| Me4tds | 3.50–3.59, d, 12H, (CH3– of Me4tds) | 41.76 (CH3– of Me4tds), 47.06 (CH3– of Me4tds), 191.76 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S of Me4tds) S of Me4tds) |
| Et4tds | 1.17–1.20, t, 6H, (CH3– of Et4tds), 1.38–1.40, t, 6H, (CH3– of Et4tds), 3.94–4.00, q, 8H, (–CH2– of Et4tds) | 11.18 (CH3– of Et4tds), 13.29 (CH3– of Et4tds), 47.25 (–CH2– of Et4tds), 51.52 (–CH2– of Et4tds), 190.85 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S of Et4tds) S of Et4tds) |
| {[BiBr(Me2DTC)2]}n (1) | 3.30, s, 12H, (CH3– of 1) | 43.31 (CH3– of 1), 199.36 (CS2 of 1) |
| {[BiBr2(Et2DTC)]}n (2) | 1.26–1.29, t, 12H, (CH3– of 2), 3.64–3.68, q, 8H, (–CH2– of 2) | 12.15 (CH3– of 2), 47.97 (–CH2– of 2), 196.62 (CS2 of 2) |
| {[BiI2(Me2DTC)]}n (3) | 3.30, s, 12H, (CH3– of 3) | 43.44 (CH3– of 3), 199.46 (CS2 of 3) |
| {[BiI(Et2DTC)2]}n (4) | 1.24–1.27, t, 12H, (CH3– of 4), 3.68–3.73, q, 8H, (–CH2– of 4) | 12.12 (CH3– of 4), 48.13 (–CH2– of 4), 198.41 (CS2 of 4) |
| {[BiI(μ2-I)(Et2DTC)2]2}n (5) | 1.25–1.28, t, 24H, (CH3– of 5), 3.66–3.71, q, 16H, (–CH2– of 5) | 12.16 (CH3– of 5), 48.10 (–CH2– of 5), 197.66 (CS2 of 5) |
The 13C-NMR spectra of Me4tms, Me4dts and Et4tds ligands show signals at 186.12 ppm, 191.76 ppm and 190.85 ppm, respectively, due to the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C(
C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) carbons (Fig. S7–S34†). The 13C(NCS2) resonance signal in the 13C-NMR spectra of 1–5 is observed at 199.36 (1), 196.62 (2), 199.46 (3), 198.41 (4) and 197.66 ppm (5) respectively, compared to similar dithiocarbamate complexes. The methyl carbons were observed at 43.31 (1), 12.15 (2), 43.44 (3), 12.12 (4) and 12.16 (5) respectively. The signals at 47.97 ppm (2), 48.13 (4) and 48.10 ppm (5) are assigned to the methylene carbons.
S) carbons (Fig. S7–S34†). The 13C(NCS2) resonance signal in the 13C-NMR spectra of 1–5 is observed at 199.36 (1), 196.62 (2), 199.46 (3), 198.41 (4) and 197.66 ppm (5) respectively, compared to similar dithiocarbamate complexes. The methyl carbons were observed at 43.31 (1), 12.15 (2), 43.44 (3), 12.12 (4) and 12.16 (5) respectively. The signals at 47.97 ppm (2), 48.13 (4) and 48.10 ppm (5) are assigned to the methylene carbons.
2.5. Crystal and molecular structures of {[BiBr(Me2DTC)2]}n (1), {[BiBr2(Et2DTC)]}n (2), {[BiI2(Me2DTC)]}n (3), {[BiI(Et2DTC)2]}n (4) and {[BiI(μ2-I)(Et2DTC)2]2}n (5)
ORTEP diagrams of 1–5 are shown in Fig. 3–7 while selected bond lengths and angles are given in Table 3.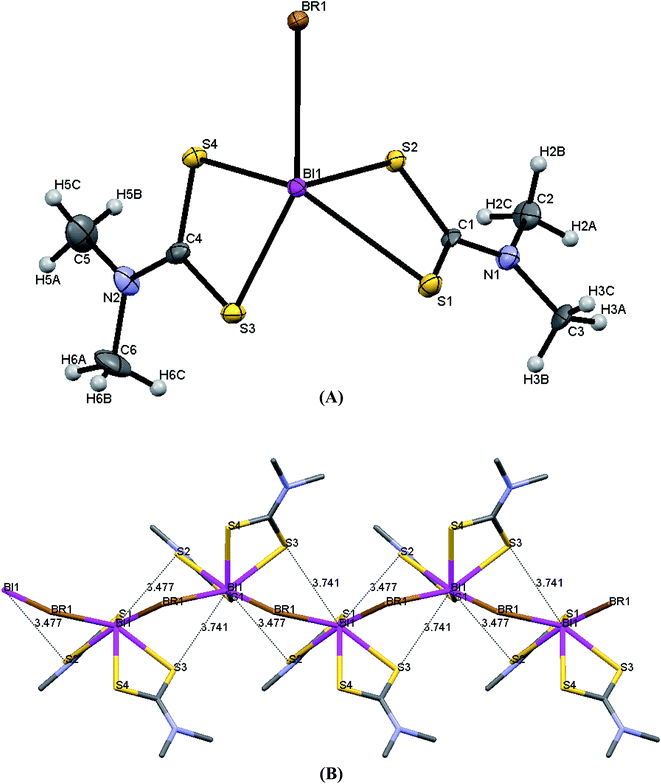 | ||
| Fig. 3 (A) ORTEP diagram together with labeling scheme of 1 (B) intermolecular μ2-S⋯Bi and μ2-Br⋯Bi interactions leading to polymerization in complex 1. | ||
 | ||
| Fig. 4 (A) ORTEP diagram together with labeling scheme of 2 (B) intermolecular μ2-S⋯Bi and μ2-Br⋯Bi interactions leading to polymerization in complex 2. | ||
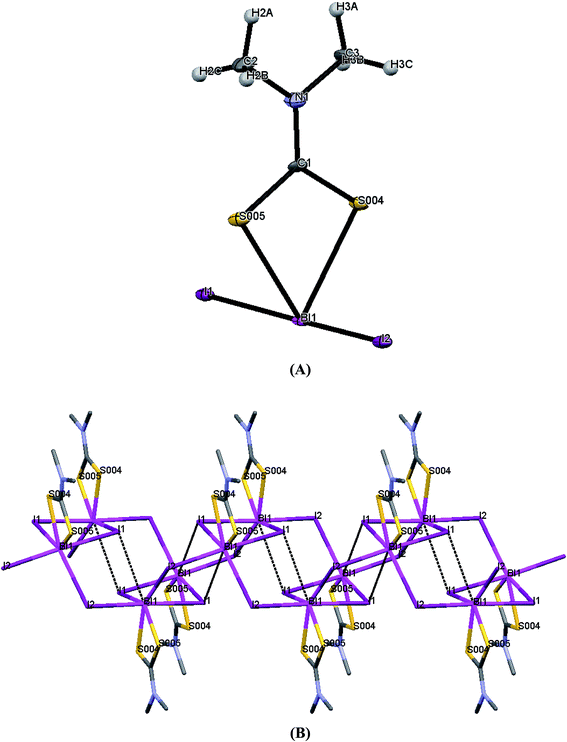 | ||
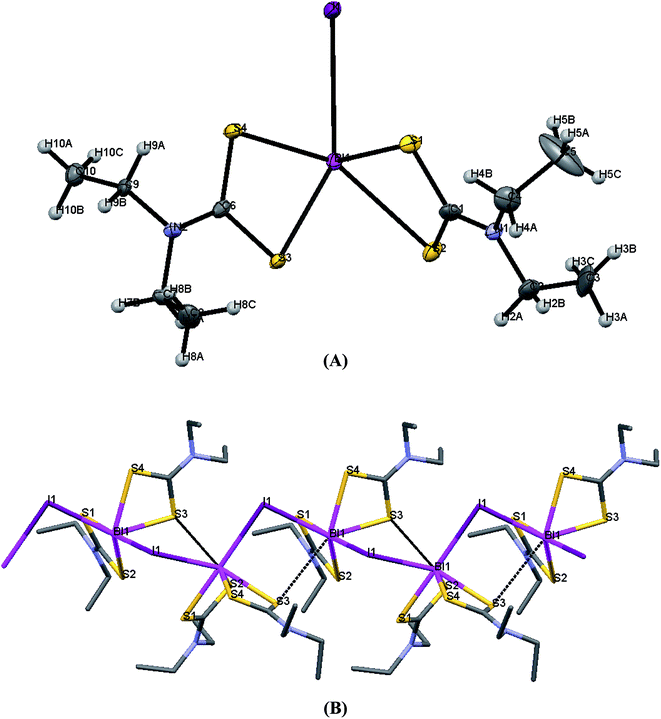
| Fig. 5 (A) ORTEP diagram together with labeling scheme of 3 (B) intermolecular μ2-I⋯Bi interactions leading to polymerization in complex 3. | ||
 | ||
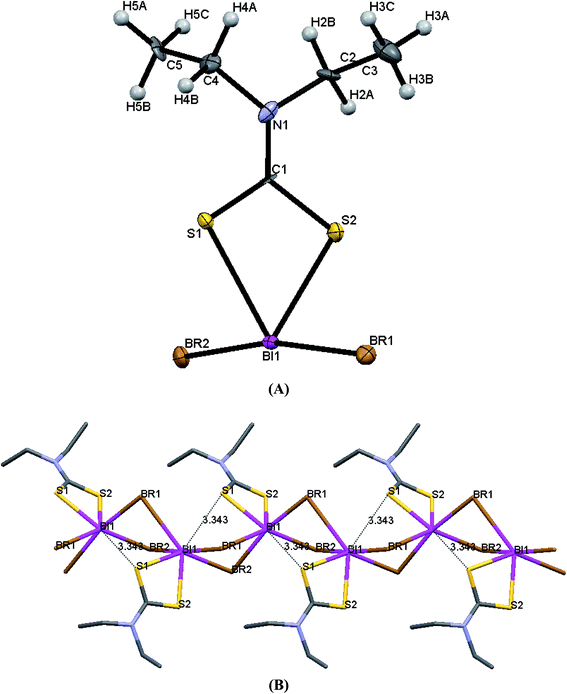
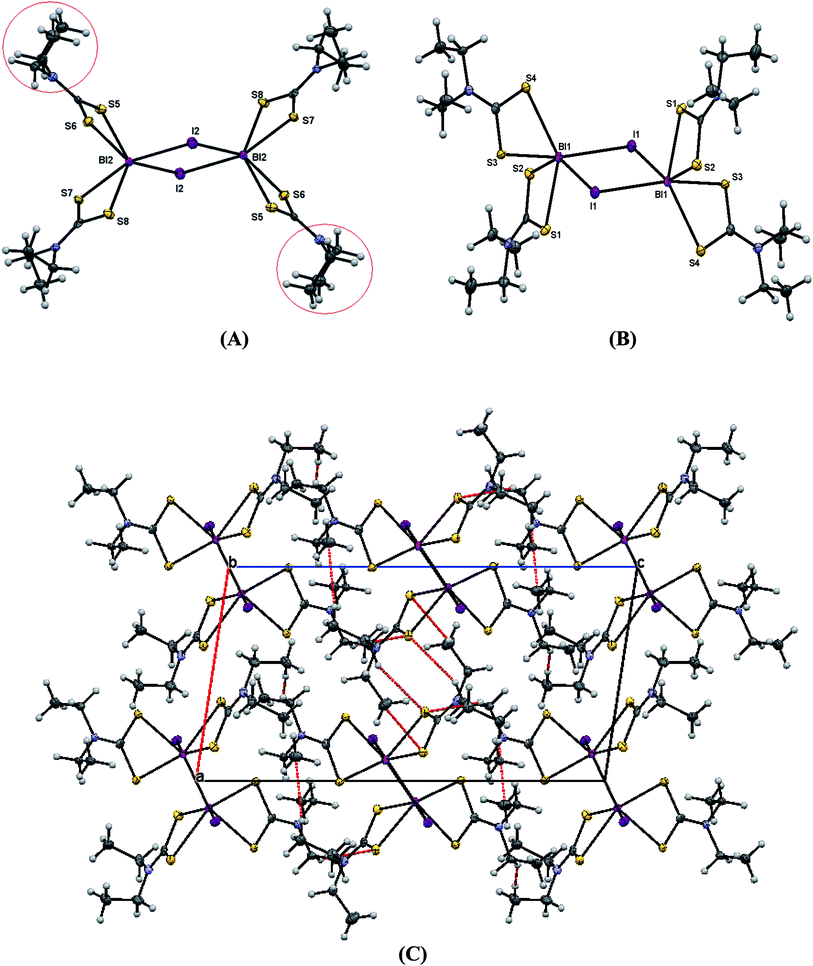
| Fig. 6 (A) ORTEP diagram together with labeling scheme of 4 (B) intermolecular μ2-S⋯Bi and μ2-I⋯Bi interactions leading to polymerization in complex 4. | ||
 | ||
| Fig. 7 (A and B) ORTEP diagram together with labeling scheme of 5 (C) unit cell of complex 5. Strong hydrogen bonds result. | ||
| 1a | 1b | 2 | 3 | 4 | 5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond length (Å) | |||||||||||||
| Bi1–Br1 | 2.9498(6) | Bi1–Br1 | 2.9564(13) | Bi1–Br1 | 2.8236(14) | Bi1–I1 | 3.0778(6) | Bi1–I1 | 3.250(2) | Bi1–I1 | 3.2682(5) | Bi2–S8 | 2.7605(19) |
| Bi1–S1 | 2.9237(17) | Bi1–S1 | 2.917(3) | Bi1–Br2 | 2.9143(14) | Bi1–I2 | 3.0825(6) | Bi1–S1 | 2.650(2) | Bi1–S1 | 2.6878(19) | Bi2–S5 | 2.7739(19) |
| Bi1–S2 | 2.6543(15) | Bi1–S2 | 2.662(3) | Bi1–S1 | 2.673(3) | Bi1–S4 | 2.6294(18) | Bi1–S2 | 2.865(3) | Bi1–S2 | 2.6650(18) | Bi2–S6 | 2.6433(18) |
| Bi1–S3 | 2.7010(15) | Bi1–S3 | 2.701(3) | Bi1–S2 | 2.604(3) | Bi1–S5 | 2.658(2) | Bi1–S3 | 2.700(2) | Bi1–S3 | 2.6404(18) | Bi2–I2 | 3.2438(6) |
| Bi1–S4 | 2.6840(15) | Bi1–S4 | 2.695(3) | Bi1–Br2_a | 3.0116(14) | Bi1–I2_a | 3.2807(6) | Bi1–S4 | 2.694(2) | Bi1–S4 | 2.8467(19) | Bi2–S7 | 2.6670(18) |
| Bi1–Br1_b | 3.1257(7) | Bi1–Br1_b | 3.1251(13) | S1–C1 | 1.722(13) | S4–C1 | 1.725(10) | Bi1–I1_b | 3.335(2) | S1–C1 | 1.730(7) | S5–C11 | 1.705(7) |
| S1–C1 | 1.703(6) | S1–C1 | 1.682(12) | S5–C1 | 1.745(9) | S1–C1 | 1.740(8) | S2–C1 | 1.730(8) | S6–C11 | 1.758(8) | ||
| S2–C1 | 1.740(6) | S2–C1 | 1.734(13) | S2–C1 | 1.683(7) | S3–C6 | 1.738(8) | S7–C16 | 1.749(8) | ||||
| S3–C4 | 1.733(6) | S3–C4 | 1.748(14) | S3–C6 | 1.752(8) | S4–C6 | 1.730(8) | S8–C16 | 1.718(8) | ||||
| S4–C4 | 1.719(6) | S4–C4 | 1.723(14) | S4–C6 | 1.709(7) | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||
| Bond angles (°) | |||||||||||||
| Br1–Bi1–S1 | 126.12(3) | Br1–Bi1–S1 | 126.07(7) | Br1–Bi1–Br2 | 168.83(4) | I1–Bi1–I2 | 172.94(2) | I1–Bi1–S1 | 81.84(4) | I1–Bi1–S1 | 77.24(4) | S5–Bi2–S8 | 139.56(6) |
| Br1–Bi1–S2 | 80.98(3) | Br1–Bi1–S2 | 80.89(7) | Br1–Bi1–S1 | 100.24(7) | I1–Bi1–S4 | 87.75(5) | I1–Bi1–S2 | 132.82(4) | I1–Bi1–S2 | 144.19(4) | I2–Bi2–S5 | 118.09(4) |
| Br1–Bi1–S3 | 146.82(4) | Br1–Bi1–S3 | 147.01(7) | Br1–Bi1–S2 | 94.03(8) | I1–Bi1–S5 | 87.70(5) | I1–Bi1–S3 | 141.16(4) | I1–Bi1–S3 | 88.85(4) | I2–Bi2–S6 | 89.93(4) |
| Br1–Bi1–S4 | 80.13(3) | Br1–Bi1–S4 | 79.94(7) | Br1–Bi1–Br2_a | 84.72(4) | I1–Bi1–I2_a | 86.00(2) | I1–Bi1–S4 | 75.04(4) | I1–Bi1–S4 | 125.60(4) | I2–Bi2–S7 | 156.53(4) |
| Br1–Bi1–Br1_b | 85.80(2) | Br1–Bi1–Br1_b | 85.85(4) | Br2–Bi1–S1 | 90.75(7) | I2–Bi1–S4 | 93.71(5) | I1–Bi1–I1_b | 94.98(1) | S1–Bi1–S2 | 67.60(6) | I2–Bi2–S8 | 90.84(4) |
| S1–Bi1–S2 | 63.88(4) | S1–Bi1–S2 | 63.65(9) | Br2–Bi1–S2 | 91.86(7) | I2–Bi1–S5 | 86.44(5) | S1–Bi1–S2 | 64.71(5) | S1–Bi1–S3 | 85.27(6) | S5–Bi2–S6 | 66.91(6) |
| S1–Bi1–S3 | 80.74(5) | S1–Bi1–S3 | 80.58(9) | Br2–Bi1–Br2_a | 87.54(4) | I2–Bi1–I2_a | 89.22(1) | S1–Bi1–S3 | 93.49(5) | S1–Bi1–S4 | 140.56(6) | S5–Bi2–S7 | 84.78(5) |
| S1–Bi1–S4 | 129.91(5) | S1–Bi1–S4 | 130.06(9) | S1–Bi1–S2 | 68.28(9) | S4–Bi1–S5 | 68.15(6) | S1–Bi1–S4 | 92.45(6) | S2–Bi1–S3 | 94.69(6) | S7–Bi2–S8 | 66.59(5) |
| Br1_b–Bi1–S1 | 137.64(3) | Br1_b–Bi1–S1 | 137.65(7) | Br2_a–Bi1–S1 | 144.03(7) | I2_a–Bi1–S4 | 148.98(5) | I1_b–Bi1–S1 | 175.79(4) | S2–Bi1–S4 | 87.71(5) | S6–Bi2–S7 | 94.98(6) |
| S2–Bi1–S3 | 97.91(5) | S2–Bi1–S3 | 98.13(10) | Br2_a–Bi1–S2 | 75.86(7) | I2_a–Bi1–S5 | 81.26(4) | S2–Bi1–S3 | 76.22(5) | S3–Bi1–S4 | 65.88(6) | S6–Bi2–S8 | 86.97(6) |
| S2–Bi1–S4 | 83.27(5) | S2–Bi1–S4 | 83.64(10) | Bi1–Br2–Bi1_b | 87.62(4) | S2–Bi1–S4 | 135.00(6) | ||||||
| Br1_b–Bi1–S2 | 157.84(4) | Br1_b–Bi1–S2 | 158.13(7) | I1_b–Bi1–S2 | 119.46(4) | ||||||||
| S3–Bi1–S4 | 66.88(4) | S3–Bi1–S4 | 67.24(10) | S3–Bi1–S4 | 66.61(6) | ||||||||
| Br1_b–Bi1–S3 | 83.56(3) | Br1_b–Bi1–S3 | 83.66(7) | I1_b–Bi1–S3 | 87.25(4) | ||||||||
| Br1_b–Bi1–S4 | 76.97(3) | Br1_b–Bi1–S4 | 76.94(7) | I1_b–Bi1–S4 | 84.04(4) | ||||||||
| Bi1–Br1–Bi1_a | 90.76(2) | Bi1–Br1–Bi1_a | 90.72(4) | ||||||||||
Complexes 1 and 4 are polymers, while their metal centers are five coordinated with distorted square pyramidal (SP) geometry in each monomeric unit. Four sulfur atoms from dithiocarbomate ligands and one halide ion are bound to Bi atom forming the building blocks of the polymer in both complexes. S1, S3 and S4 atoms of the dithiocarbamate ligands and Br atom in 1, S2, S3 and S4 atoms of the dithiocarbamate ligands and I atom in 4 lie in a plane, while atom S2 in 1 and atom S1 in 4 associated to dithiocarbamate ligand fills the apical position due to perpendicular arrangement of the ligand to the basal plane. The dithiocarbamate ligands are anisobidentate μ2-bridging in both complexes. Two μ2-S⋯Bi (Bi1⋯S2 = 3.477 and Bi1⋯S3 = 3.741 Å in 1a, Bi1⋯S2 = 3.474 and Bi1⋯S3 = 3.731 Å in 1b) and one μ2-Br⋯Bi (Bi1⋯Br1 = 3.126 Å in 1a, Bi1⋯Br1 = 3.125 Å in 1b) strong intermolecular interactions lead to a polymeric assembly with distorted square antiprismatic geometry around the Bi(III) ion in complex 1 (the sum of van der Waals radii lies between 4.1 and 5.58 Å for Bi–S and between 4.2 and 5.62 Å for Bi–Br25). One μ2-S⋯Bi (Bi1⋯S3 = 3.424 Å) and one μ2-I⋯Bi (Bi1⋯I1 = 3.335 Å) strong intermolecular interaction lead to a polymeric assembly with pentagonal bipyramidal (PBP) geometry around the Bi(III) ion in case of 4 (The sum of van der Waals radii lies between 4.4 and 5.74 Å for Bi–I25). Two stronger metal–sulfur bonds, with shorter Bi–S bond lengths (Bi1–S2 = 2.6543(15), Bi1–S4 = 2.6840(15) Å (1a), Bi1–S2 = 2.662(3), Bi1–S4 = 2.695(3) Å (1b) and Bi1–S1 = 2.650(2), Bi1–S4 = 2.694(2) Å (4)) and two weaker bonds with longer Bi–S distances (Bi1–S1 = 2.9237(17), Bi1–S3 = 2.7010(15) Å (1a), Bi1–S1 = 2.917(3), Bi1–S3 = 2.701(3) Å (1b) and Bi1–S2 = 2.865(3), Bi1–S3 = 2.700(2) Å (4)) are formed. The Bi–X bond lengths of the terminal halide atoms are Bi1–Br1 = 2.9498(6) Å in 1a, Bi1–Br1 = 2.9564(13) Å in 1b and Bi1–I1 = 3.250(2) Å in 4. The equatorial angles in 1a, 1b and 4 are: Br1–Bi1–S1 = 126.12(3)°, Br1–Bi1–S4 = 80.13(3)°, S1–Bi1–S3 = 80.74(5)°, S3–Bi1–S4 = 66.88(4)° (1a), Br1–Bi1–S1 = 126.07(7)°, Br1–Bi1–S4 = 79.94(7)°, S1–Bi1–S3 = 80.58(9)°, S3–Bi1–S4 = 67.24(10)° (1b) and I1–Bi1–S2 = 132.82(4)°, I1–Bi1–S4 = 75.04(4)°, S2–Bi1–S3 = 76.22(5)°, S3–Bi1–S4 = 66.61(6)° (4) indicating high deviation from their ideal geometry. These deviations from the 90° of the ideal square pyramidal geometry are due to the repulsions between the free electrons pair located on the bismuth and those of the covalent Bi–X bonds (X: S, Br or I) according to the Valence Shell Electron Pair Repulsion (VSEPR) theory. In complex 4, there are two different diethyldithiocarbamate ligands, one with cis and the second with trans disposition of the methyl carbons of ligands (Scheme 2) (C1–N1–C2–C3 = 85.4(8)°, C1–N1–C4–C5 = −97.8(9)°, C6–N2–C7–C8 = 93.9(8)°, C6–N2–C9–C10 = 85.5(8)°).
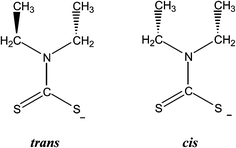 | ||
| Scheme 2 Isomers observed in diethyldithiocarbamate ligand (defined based on the CCNC torsion angles). | ||
Complex 2 is polymer and the metal center is four coordinated with pseudo-trigonal bipyramidal geometry in each monomeric unit. Two sulfur atoms from dithiocarbomate ligand and two bromide ions are bound to Bi atom forming building blocks for polymer. The two bromide atoms are trans to each other in monomeric unit. The dithiocarbamate ligand is anisobidentate μ2-bridging. Two μ2-Br⋯Bi (Bi1⋯Br1 = 3.293 Å, Bi1⋯Br2 = 3.012 Å) and one μ2-S⋯Bi (Bi1⋯S1 = 3.343 Å) strong intermolecular interactions lead to a polymeric assembly with pentagonal bipyramidal geometry (PBP) around the bismuth(III) ion.25 The terminal Bi–S bond lengths are Bi1–S2 = 2.604(3) Å and Bi1–S1 = 2.673(3) Å. The Bi–Br bond lengths of the terminal bromide atoms are Bi1–Br1 = 2.8236(14) Å and Bi1–Br2 = 2.9143(14) Å. In complex 2, there is one diethyldithiocarbamate ligand with the methyl groups to be in trans disposition (C1–N1–C2–C3 = −89.6(15)°, C1–N1–C4–C5 = −98.5(14)°).
Complex 3 exists as polymer in the solid state. The geometry around the metal center in each monomeric unit is pseudo-trigonal bipyramidal geometry. Two sulfur atoms from dithiocarbomate ligand and two iodide ions are bound to Bi atom. The two iodide atoms are trans to each other in monomeric unit and dithiocarbamate ligand is anisobidentate. Strong intermolecular interactions between μ2-I and Bi atom (Bi1⋯I1 = 3.366(2) Å) lead to a dimeric assembly with square pyramidal geometry (SP) around the bismuth(III) ion and intermolecular interactions between μ2-I and Bi atoms in each dimeric units (Bi1⋯I2_a = 3.2807(6) Å) lead to a polymeric assembly with pentagonal bipyramidal geometry around the Bi(III) ion.25 The terminal Bi–S bond lengths are Bi1–S4 = 2.6294(18) Å and Bi1–S5 = 2.658(2) Å. The Bi–I bond lengths of the terminal iodide atoms are Bi1–I1 = 3.0778(6) Å and Bi1–I2 = 3.0825(6) Å.
In the crystal structure of 5 there are two dimeric molecules, one with two cis and two trans-dithiocarbamate ligands and the second with four trans-dithiocarbamate ligands around coordination centers. Four sulfur atoms from dithiocarbamate ligands and one iodide ion are bound to bismuth ions forming the building block of the dimmers. The geometry around the metal center in each monomeric unit is square pyramidal geometry. Two strong intramolecular interactions between μ2-I and Bi atoms (Bi1⋯I1 = 3.2682(5) Å, Bi2⋯I2 = 3.2438(6) Å) lead to dimerism with distorted octahedral geometry around bismuth(III) ion.25 Furthermore intermolecular hydrogen bonding leads to polymeric assembly in case of 5 (S1⋯H2B = 2.999(8) Å, S2⋯H4B = 3.002(8) Å, S3⋯H7A = 2.983(7) Å). The terminal Bi–S bond lengths are Bi1–S1 = 2.6878(19) Å, Bi1–S2 = 2.6650(18) Å, Bi1–S3 = 2.6404(18) Å, Bi1–S4 = 2.8467(19) Å, Bi2–S5 = 2.7739(19) Å, Bi2–S6 = 2.6433(18) Å, Bi2–S7 = 2.6670(18) Å, Bi2–S8 = 2.7605(19) Å. The equatorial angles in 5 are: I1–Bi1–S1 = 77.24(4)°, I1–Bi1–S4 = 125.60(4)°, S1–Bi1–S2 = 67.60(6)°, S2–Bi1–S4 = 87.71(5)°, I2–Bi2–S5 = 118.09(4)°, I2–Bi2–S8 = 90.84(4)°, S5–Bi2–S7 = 84.78(5)°, S7–Bi2–S8 = 66.59(5)° indicating high deviation from their ideal geometry. These deviations from the 90° of the ideal square pyramidal geometry are due to the repulsions between the free electrons pair located on the bismuth and those of the covalent Bi–I bonds in accordance to the Valence Shell Electron Pair Repulsion (VSEPR) theory. In complex 5, there are two different diethyldithiocarbamate ligands, one with cis and the other four with trans disposition of the methyl carbons of ligands (Scheme 2) (C1–N1–C2–C3 = −94.0(8)°, C1–N1–C4–C5 = −98.0(8)°, C6–N2–C7–C8 = −96.9(8)°, C11–N3–C12–C13 = 87.9(8)°, C11–N3–C14–C15 = −89.4(8)°, C16–N4–C17–C18 = −94.1(8)°, C16–N4–C19–C20 = −91.3(8)°).
The Bi–S bond distances are varied from 2.604 to 2.924 Å in complexes 1–5 and they are in agreement with those values previously found for similar complexes.11,20 The Bi–Br bond distances are varied from 2.824 to 2.950 Å in complexes 1 and 2, while the Bi–I bond distances are varied from 3.078 to 3.268 Å in complexes 3–5. Both Bi–Br and Bi–I distances found in complexes 1–5 are also in agreement with those reported earlier.11,20 These distances are shorter than the sum of bismuth and sulfur or bromide or iodide van der Waals radii.25
The C–S bonds are varied between 1.683 and 1.758 Å in complexes 1–5; these distances are in the range of the free tetramethylthiuram monosulfide (1.655 Å), tetramethylthiuram disulfide (1.647 Å) and tetraethylthiuram disulfide (1.643 Å). The C–S single bonds in free ligands are 1.787–1.807 Å for tetramethylthiuram monosulfide, 1.805 Å for tetramethylthiuram disulfide and 1.820–1.825 Å for tetraethylthiuram disulfide.26
2.6. Biological studies
The metallodrugs 1–5 were evaluated for their antiproliferative activity against adenocarcinoma cancerous cells lines: MCF-7 (breast) and HeLa, (cervix) with sulforhodamine B (SRB) assay for a period of 48 h (Table 4). The IC50 values of 1–5 against HeLa cells lie in the range of nanomolars, 0.05–0.3 μM, while against MCF-7 cells in the range of 0.05–0.1 μM. The most promising complexes are 4 and 5 with IC50 value equal to 0.05 μM (50 nM) against both adenocarcinoma cell lines. The complexes exhibit stronger activity than cisplatin which is up to 100 times (4 and 5) against HeLa cells and 136 times (4) against MCF-7 cells. The MCF-7 cells are more sensitive in 1–5 than HeLa cells (Table 4). Especially against MCF-7 cells bismuth complexes (Table 4) have shown comparable or even better activity than the other standard anti-cancer drugs such as tamoxifen11 an antiestrogen drug which inhibits the growth of MCF-7 cells by blocking the steroid receptors (ER-α and ER-β). The steroid receptors (ER-α and ER-β) are located in MCF-7 cells,.11 In contrast HeLa cells are devoid of estrogen receptors (ERs).11 The activity of 1–5 is in accordance with the bismuth(III) complexes, {[BiCl(Me2DTC)2]n} (Me2DTCH = dimethyldithiocarbamate) and {[Bi(Et2DTC)3]2} (Et2DTCH = diethyldithiocarbamate) (Table 4).11 Among bismuth complexes, the higher activity against MCF-7 and HeLa cells is shown by the complex {[BiCl(Me2DTC)2]n} (IC50 = 0.02 μM) and 4 (IC50 = 0.1 μM), respectively. Although more data are needed, it is however noticeable, that the halogen type affects the bioactivity of the compounds in case of MCF-7 cells. Thus, chloride derivatives generally show lower IC50 values (higher activity) than iodide ones (Table 4). Moreover, the complexes containing methyl substituted dithiocarbamates exhibit stronger activity than those of ethyl ones. However, this is not observed in the case of HeLa cells, where the iodide derivatives of ethyl substituted dithiocarbamate complexes are more active. This might be attributed to the different origin of the cancer cells tissues.| Compounds | Volume (A3) | Contacts (%) | IC50 (μM) | Ref. | ||
|---|---|---|---|---|---|---|
| HeLa | MCF-7 | MRC-5 | ||||
| a This work, Me2DTCH = dimethyldithiocarbamate, Et2DTCH = diethyldithiocarbamate. | ||||||
| 1a | 330.78 | 64.6 | 0.2 ± 0.01 | 0.08 ± 0.01 | 0.25 ± 0.01 | a |
| 2 | 284.11 | 59.4 | 0.2 ± 0.01 | 0.08 ± 0.006 | 0.32 ± 0.02 | a |
| 3 | 258.42 | 47.0 | 0.3 ± 0.02 | 0.1 ± 0.003 | 0.29 ± 0.01 | a |
| 4 | 439.5 | 73.2 | 0.1 ± 0.01 | 0.05 ± 0.002 | 0.18 ± 0.01 | a |
| 5 | 445.83 | 75.1 | 0.05 ± 0.006 | 0.07 ± 0.008 | 0.15 ± 0.02 | a |
| {[BiCl(Me2DTC)2]n} (6) | 414.73 | 64.9 | 0.33 ± 0.03 | 0.023 ± 0.003 | 11 | |
| {[Bi(Et2DTC)3]2} (7) | 1207.07 | 86.7 | 0.19 ± 0.02 | 0.043 ± 0.008 | 11 | |
| Cisplatin | 10.0 | 6.8 ± 0.3 | 11 | |||
| Tamoxifen | 0.0455 | 11 | ||||
The toxicity of the complexes is also tested, against normal human fetal lung fibroblast cells (MRC-5) cells (Table 4). The IC50 values lie between 0.15 and 0.23 μM (toxicity order: 5 > 4 > 1α > 3 > 2). Complexes 2, 4 and 5 show selectivity against cancerous cell lines than normal (Table 4).
Since ERs are located in MCF-7, in contrast to HeLa cells the estrogenic effect of 1–5 on MCF-7 cells, was studied after 5 days of cell culture using methylene blue assay.27a–c Methylene blue modulates the actions of estrogens, helps localize occult breast tumor and interferes with the estrogen-receptor protein.27e,f The phenol red-free culture medium is used, since phenol red resemblances to some nonsteroidal estrogens having weak estrogen agonist activity.27g,h Thus, the absence of phenol red in the cell medium secures the avoidance potential estrogen-like effects of this compound.27
The estrogenic activity is calculated by the percentage of (Acontrol − Acomplex)/Acontrol. The estrogenic activity of the compounds is: 14.5 (1), 7.0 (2), 1.0 (3), 48.3 (4) and 55.1 (5)%, respectively. Thus, 1–5 show estrogenic proliferative effect on MCF-7 cells at their IC50 values with 4 and 5 to exhibit higher effect among them. This might be attributed to their docking pocket which is similar to 4-hydroxytamoxifen while they exhibit the lower docking score (see docking studies).
2.7. Docking studies
In order to ascertain the influence of the estrogen receptors in the activity of 1–7 (Table 4) towards MCF-7 cells docking studies were performed. ER's are proteins which belong to the superfamily of steroid/thyroid hormone nuclear receptors and are able to bind estrogens with high affinity.28a They are ligand-inducible intracellular transcription factors which modulate gene expression in many tissues. The physiological effects of estrogen are expressed via two main ER isoforms, ER-α and ER-β which exhibit different tissue distribution and signaling response.28b ER-α is over expressed in breast cancer and thus plays a major role in the cancerous cell proliferation. Contrary, ER-β has found to inhibit proliferation, migration and invasion of breast cancers cells in in vitro studies.28c–dIn hormone-dependent breast cancers, ERs are present in tumour cells (ER+). Tamoxifen is a non-steroidal anti-estrogenic drug which is extensively used as an ER antagonist to treat hormone-responsive human breast cancers in pre- and post-menopausal women28e The 4-hydroxytamoxifenis the active metabolite of the pro-drug tamoxifen28f and its anti-estrogenic effect is due to its competitive binding to ER-α. The dimethylaminoethoxy side chain of the 4-hydroxytamoxifensignificantly increases the binding affinity by inducing a stabilizing interaction with the Asp351 (anionic carboxylate site) residue of ER-α.28g The crystal structure of the ligand binding domain (LBD)28f also shows strong hydrogen bonding interactions with Glu353 and Arg394. The interaction with Asp351 prevents the association of Helix 4 with Helix 12 (ref. 28h) while the hydrogen bonding network further stabilizes the binding of the ligand. Nevertheless, it has been suggested that the antiproliferative activity against human breast cancer cells (MCF-7) is not altered when the –CH2CH3 moiety in tamoxifen is replaced by –CH3.28i Moreover, good binding affinity and noteworthy antiproliferative activity (MFC-7) was marked when an organometallic moiety has replaced the amino side chain of hydroxytamoxifen.28h The combination of the latter finding as well as the remarkable activity of complexes against MCF-7 led us to perform molecular docking experiments in the LBD of ER-α structure crystallized with 4-hydroxytamoxifen (PBD ID: 3ERT). Validation docking was performed and the root mean square deviation (RMSD) between the co-crystallized and the docked ligand and was found to be 1.2 Å. All complexes (but 7) (Table 4) are able to bind in hydrophobic binding pocket of the 4-hydroxytamoxifen albeit with less scoring energy than that of the drug (docking score: −106 in arbitrary units). Only 3, 4 and 5 interact with Asp351 through electrostatic interactions but only 4 and 5 adopt the drug orientation. 1, 2, and 6 are accommodated in the vicinity of Glu353 and Glu419 having similar interaction energies which are lower than those of 4 and 5 (docking score: −65 and −73, respectively). In Fig. 8 the hydrophobic surface of 4 (blue) and 5 (green) is shown on top of the 4-hydroxytamoxifen structure. In strictly structural terms, complex 5 resembles 4-hydroxytamoxifen by having similar hydrophobic surface dimensions. Thus, 5 can possibly induce anti-estrogenic effects by adopting a similar pose into the binding site. On the other hand, docking in the site of the dimethylaminoethoxy side chain of 4-hydroxytamoxifen is energetically favored for the smaller 4 yielding significant activity.
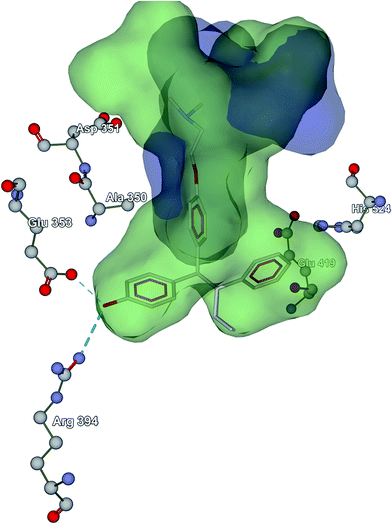 | ||
| Fig. 8 The hydrophobic volume of 4 (blue) and 5 (green) on top of the 4-hydroxytamoxifen structure in its binding site. | ||
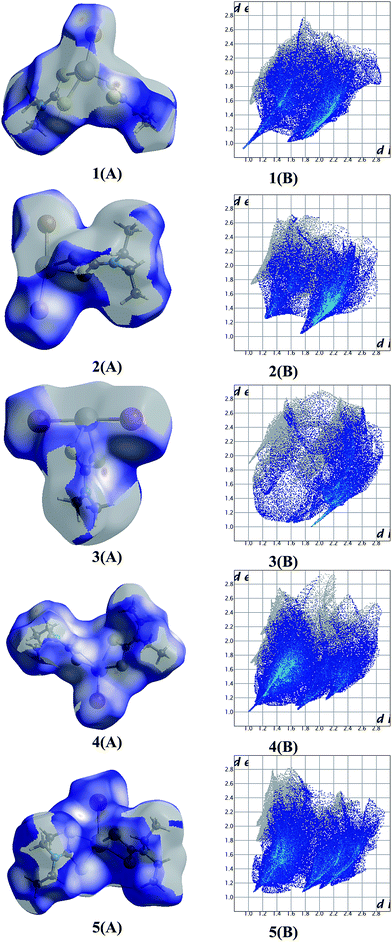
2.8. Hirshfeld surface analysis
The Hirshfeld surface is defined as the volume of space where the molecule electron density exceeds that from all neighboring molecules.29 Hirshfeld surfaces also provide a three-dimensional picture of the close contacts in a crystal structure and these contacts can be summarized in a 2D fingerprint plot.29Fig. 9 shows the dnorm surfaces of the complexes 1–5 (A). The nature of the intermolecular interactions was clarified by the 2D fingerprint plot (B).29 The close contacts of all elements inside the area with the outer hydrogen atoms were calculated for the complexes 1–5 are: 64.6% (1), 59.4% (2), 47% (3), 73.2% (4) and 75.1% (5) respectively. The results show that the lower the contacts between molecules in the compounds, the greater the bioactivity against adenocarcinoma cells is (Fig. S35 and S36†). This is in accordance to our earlier findings for antimony dithiocarbamate compounds where the complexes with higher activity against MCF-7 cells exhibit low H-all intermolecular atoms interactions.29 Moreover similar trends are observed for the bioactivity of the bismuth compounds vs. volumes.
2.9. Computational studies
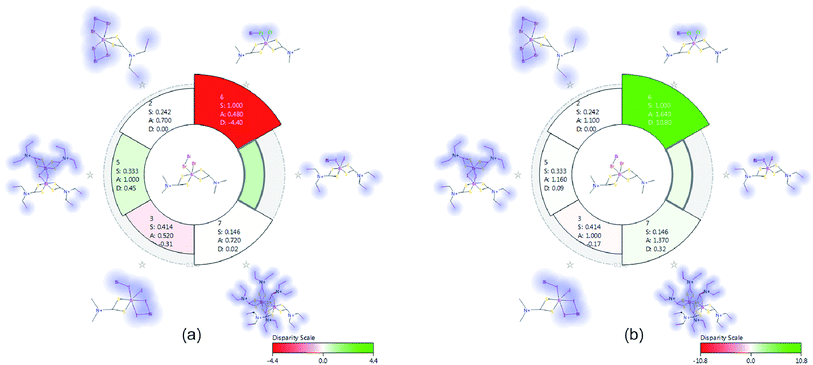
Structure–activity relationship (SAR) studies were performed for all complexes using 2D topological based disparity analysis. This can be characterized as pseudo-scaffold-hopping study of this class of bismuth complexes which exhibit the hydrophobic Bi–S2–C ring. Nevertheless, apart from this core moiety, the seven compounds (Table 4) show noticeable discrepancy regarding their structure and their basic descriptors (like MW, TPSA, SlogP etc.) while their activity expressed by half maximum inhibitory concentrations (IC50) towards HeLa and MCF-7 cell lines is noteworthy. As a first approximation, we evaluated the SAR of these compounds using Activity Miner (Cresset-UK).30a,b Activity Miner compares structural and field properties of pairs of molecules trying to assess large variations in activity using the following notion of disparity:| disparity = f(Δactivity/(1 − similarity)) |
Thus, the higher disparity is observed for similar molecules with significant activity difference. All complexes in this study feature the Bi–[S2–C–N–C2]x (x = 1, 2) moiety and through the “disparity” approach we tried to assess the topological similarity vs. inhibition activity (IC50). We constructed the disparity matrix (Fig. 10) using the 2D similarity FCFP6 fingerprint. Extended-connectivity fingerprints (ECFPs) and their variants functional-connectivity fingerprints (FCFPs) are topological entities specifically developed for structure–activity modeling.30c ECFPs are circular fingerprints originally proposed for discriminating isomorphs30d and work by assigning an atom identifier for each heavy (non-hydrogen) atom in the molecule based on parameters like atomic number, charge, hydrogen count, etc. A functional-class rule for atoms properties relating to ligand binding (hydrogen-bond acceptor or donor, polarity, aromaticity, etc.) is assigned in FCFPs exposing the pharmacophore role of the atoms.
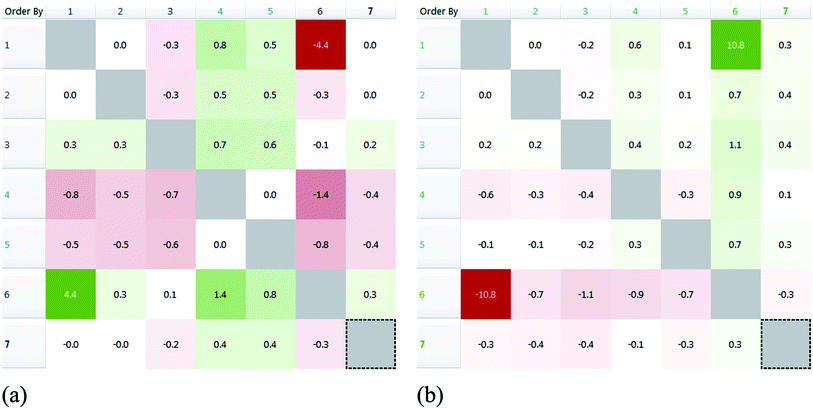 | ||
| Fig. 10 Disparity matrices with pairwise comparisons for IC50 (HeLa (a) and MCF-7 (b)) activity values. | ||
In Fig. 10, pairwise comparisons are depicted as red or green elements of a symmetrical matrix corresponding to decrease or increase in activity, respectively. The shading is analogous to the degree of disparity (i.e. darker shading means higher disparity).
Complexes behave differently in vitro towards different cancer cell lines: 4 and 5 exhibit the highest potency against HeLa and 6 against MCF-7. From the disparity matrix we can conclude that complexes 1 and 6 (Table 4) feature a steep activity cliff (i.e. significant disparity) due to their structural similarity. However, the activity is reversed between 1 and 6 for the examined cell lines and 6 is more effective against MCF-7 while 1 against HeLa (Fig. 11). This finding underlines the significance of the halogen atoms in the coordination sphere of the metal ion. This is in agreement with the results obtained from cell screening where the halogen type is affecting on the bioactivity of the complex against MCF-7. In contrast, compound 4 does not show a significant activity cliff (Fig. 11) although it is structurally similar to 1 and 6; either the ethyl-terminal groups or the iodine atoms have probably a negative effect in activity for this class of compounds.
3. Conclusions
Five new bismuth(III) halide complexes (BiX3, X = Br or I) of formulae {[BiBr(Me2DTC)2]n} (1), {[BiBr2(Et2DTC)]n} (2), {[BiI2(Me2DTC)]n} (3), {[BiI(Et2DTC)2]n} (4) and {[BiI(μ2-I)(Et2DTC)2]2}n (5) were synthesized. Alterations in preparation of 1 by the use of different processor reagents (Me4tms instead of Me4tds) and solvation effect (Scheme 1), lead to same molecular forms in the solid state (1a and 1b). On the other hand in preparation of 4 and 5 by using the same processor reagent (Et4tds) and solvation effect, different molecular forms in the solid state were obtained with metal–ligand stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for 4 and 1
2 for 4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 5. Reactions of thiuram monosulfides or disulfides with bismuth(III) halide (BiX3; X: Cl, Br or I) lead to ligand reduction with concomitant degradation to dithiocarbamate ligands. Building blocks of [BiX(L)2] units with square pyramidal geometry (X: Br or I, L: dithiocarbamate ligands, complexes 1, 4 and 5) and [BiX2(L)] units with pseudo trigonal bipyramidal geometry (X: Br or I, L: dithiocarbamate ligands, complexes 2 and 3) around bismuth(III) ions are link each other to form either a polymeric chain in case of 1, 2, 3 and 4 or dimmers complexes in case of 5.
1 for 5. Reactions of thiuram monosulfides or disulfides with bismuth(III) halide (BiX3; X: Cl, Br or I) lead to ligand reduction with concomitant degradation to dithiocarbamate ligands. Building blocks of [BiX(L)2] units with square pyramidal geometry (X: Br or I, L: dithiocarbamate ligands, complexes 1, 4 and 5) and [BiX2(L)] units with pseudo trigonal bipyramidal geometry (X: Br or I, L: dithiocarbamate ligands, complexes 2 and 3) around bismuth(III) ions are link each other to form either a polymeric chain in case of 1, 2, 3 and 4 or dimmers complexes in case of 5.
The metal–drugs 1–5 were evaluated for their antiproliferative activity against adenocarcinoma cancerous cells lines: MCF-7 (breast) and HeLa, (cervix) (Table 4). The MCF-7 cells are more sensitive in 1–5 than HeLa cells (Table 4). The most promising complexes are 4 and 5 with IC50 value (0.05 μM) against both adenocarcinoma cell lines (Table 4). This might be attributed in the blocking of the steroid receptors (ER-α and ER-β) which are present in MCF-7 cells but not in HeLa cells. Complexes 1–5 show estrogenic proliferative effect on MCF-7 cells at their IC50 values with 4 and 5 to exhibit higher effect among them as it is evidenced by methylene blue assay. This is further confirmed by docking studies (Fig. 8). Moreover the bismuth compounds studied here (Table 4) exhibit comparable or even better activity than the tamoxifen11 (an antiestrogen drug) which inhibits the growth of MCF-7 cells by blocking the steroid receptors (ER-α and ER-β). The complexes, also, exhibit stronger activity than cisplatin which is up to 100 times (4 and 5) against HeLa cells and 136 times (4) against MCF-7 cells.
The seven compounds (Table 4) show noticeable discrepancy regarding their structure and their basic descriptors (like MW, TPSA, SlogP etc.) while their activity expressed by half maximum inhibitory concentrations (IC50) towards HeLa and MCF-7 cell lines is noteworthy. This study underlines the significance of the halogen atoms in the coordination sphere of the metal ion, in agreement with the results obtained from cell screening where the halogen type is affecting on the bioactivity of the complex against MCF-7. Moreover, the ethyl-terminal groups or the iodine atoms have probably a negative effect in activity for this class of compounds. The intermolecular hydrogen bonding interactions influence the mechanism of action of these compounds. The complexes with higher activity against MCF-7 cells exhibit low H-all intermolecular atoms interactions.
4. Experimental
4.1. Materials and instruments
All solvents used were of reagent grade; bismuth(III) bromide (Aldrich), bismuth(III) iodide (Aldrich), tetramethylthiuram monosulfide (Aldrich), tetramethylthiuram disulfide (Aldrich) and tetraethylthiuram disulfide (Aldrich) were used with no other purification prior to use. Elemental analyses for C, H, N, and S were carried out with a Carlo Erba EA MODEL 1108 elemental analyzer. Melting points were measured in open tubes with a STUART SMP10 scientific apparatus and are uncorrected. FT-IR spectra were recorded in the 4000–400 cm−1 region with Bruker Optics, Vertex 70 FT-IR spectrometer using ATR techniques. Micro Raman spectra (64 scans) were recorded at room temperature using a low-power (∼30 mW) green (514.5) mm laser on a Renishaw InVia spectrometer set at 2.0 resolution. 1H and 13C NMR spectra were recorded with a Varian Unity Inova 500 MHz spectrometer for complexes 1 and 2, with a Bruker Ultrashield 400 NMR instrument for complexes 3–5 in DMSO-d6 with chemical shifts given in parts per million referenced to internal TMS (H). Thermal Gravimetry-Differential Thermal Analysis (TG-DTA) of complexes were carried out on a Seiko SII TG/DTA 7200 apparatus under N2 flow (50 cm3 min−1) with a heating rate of 10 °C min−1.4.2. Synthesis and crystallization of {[BiBr(Me2DTC)2]}n (1), {[BiBr2(Et2DTC)]}n (2), {[BiI2(Me2DTC)]}n (3), {[BiI(Et2DTC)2]}n (4) and {[BiI(μ2-I)(Et2DTC)2]2}n (5)
1: yellow crystals, yield: 53% (method A) and 71% (method B), melting point: 169–171 °C, elemental anal. calc. for C6H12BiBrN2S4, C, 13.61; H, 2.29; N, 5.29; S, 24.23, found: C, 13.42; H, 2.31; N, 5.24; S, 24.21. IR (cm−1): 1514s, 1379s, 1238m, 1144m, 1130m, 1039m, 964s, 877w, 831w, 717w, 706w, 619w, 565s, 444s.
2: yellow crystals, yield: 74%, melting point: 234–237 °C, elemental anal. calc. for C5H10BiBr2NS2, C, 11.61; H, 1.95; N, 2.71; S, 12.40, found: C, 11.54; H, 1.98; N, 2.67; S, 12.44. IR (cm−1): 2976w, 2964w, 2866w, 2361w, 2341w, 1525s, 1452s, 1433s, 1379m, 1344m, 1269m, 1192m, 1149m, 1093m, 1072m, 1057m, 989w, 976w, 906m, 839m, 777m, 737w, 719w, 702w, 557s, 484m, 467w, 405m.
3: orange crystals, yield: 76%, melting point: 272–274 °C, elemental anal. calc. for C3H6BiI2NS2, C, 6.18; H, 1.04; N, 2.40; S, 11.00, found: C, 6.12; H, 1.08; N, 2.42; S, 10.94. IR (cm−1): 2924w, 1907w, 1520s, 1381s, 1234m, 1142m, 1126m, 1038m, 955w, 877w, 841w, 806w, 756w, 727w.
4: yellow crystals, yield: 68%, melting point: 162–164 °C, elemental anal. calc. for C10H20BiIN2S4, C, 18.99; H, 3.19; N, 4.43; S, 20.28, found: C, 18.85; H, 3.23; N, 4.38; S, 20.21. IR (cm−1): 2974w, 2928w, 2866w, 2361w, 2341w, 1502s, 1483s, 1421s, 1348m, 1290w, 1263s, 1198s, 1140m, 1061m, 1011w, 984m, 908m, 837m, 771m, 669w, 607w.
5: orange crystals, yield: 74%, melting point: 166–169 °C, elemental anal. calc. for C40H80Bi4I4N8S16, C, 18.99; H, 3.19; N, 4.43; S, 20.28, found: C, 18.83; H, 3.21; N, 4.48; S, 20.14. IR (cm−1): 2972w, 2928w, 2359w, 1497s, 1427s, 1375m, 1350m, 1269s, 1198m, 1144m, 1074m, 995w, 978w, 904w, 839m, 777w, 607w.
4.3. X-ray structure determination
Intensity data for the crystals of 1–5 were collected on an Oxford Diffraction CCD instrument, using graphite monochromated Mo radiation (λ = 0.71073 Å). Cell parameters were determined by least-squares refinement of the diffraction data from 25 reflections.31 All data were corrected for Lorentz-polarization effects and absorption.31,32 The structures were solved with direct methods with SHELXS97 (ref. 33) and refined by full-matrix least-squares procedures on F2 with SHELXL97.34 All non-hydrogen atoms were refined anisotropically, hydrogen atoms were located at calculated positions and refined via the “riding model” with isotropic thermal parameters fixed at 1.2 (1.3 for CH3 groups) times the Ueq value of the appropriate carrier atom. Significant crystal data are given in Table 5.| 1a | 1b | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Empirical formula | C6H12BiBrN2S4 | C6H12BiBrN2S4 | C5H10BiBr2NS2 | C3H6BiI2NS2 | C20H40Bi2I2N4S8 | C10H20BiIN2S4 |
| Cryst syst | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/c | P21/c | P21/c | C2/c | P21/c | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 10.2109(5) | 10.2180(6) | 10.0768(6) | 23.8005(12) | 11.845(5) | 9.4033(3) |
| b (Å) | 16.3665(7) | 16.3552(9) | 14.7660(9) | 11.1504(6) | 18.266(5) | 11.2321(4) |
| c (Å) | 8.2545(4) | 8.2644(5) | 7.8460(5) | 8.0446(5) | 8.659(5) | 17.6307(6) |
| α (deg) | 90 | 90 | 90 | 90 | 90 | 93.566(3) |
| β (deg) | 101.026(4) | 100.927(6) | 91.716(5) | 92.625(5) | 106.904(5) | 98.144(3) |
| γ (deg) | 90 | 90 | 90 | 90 | 90 | 96.906(3) |
| V (Å3) | 1354.00(11) | 1356.08(14) | 1166.91(12) | 2132.7(2) | 1792.5(14) | 1824.02(11) |
| Z | 4 | 4 | 4 | 8 | 4 | 4 |
| T (K) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) |
| ρcalcd (g cm−3) | 2.597 | 2.593 | 2.943 | 3.632 | 2.343 | 2.303 |
| μ (mm−1) | 16.6 | 16.5 | 22.3 | 81.1 | 12.0 | 11.8 |
| Tot., uniq. data, R(int) | 8252, 2370, 0.050 | 4714, 2377, 0.041 | 4395, 2049, 0.041 | 6546, 1914, 0.065 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153, 3152, 0.074 153, 3152, 0.074 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422, 6419, 0.033 422, 6419, 0.033 |
| Observed data [I > 2.0 sigma(I)] | 2136 | 2079 | 1692 | 1854 | 2664 | 5902 |
| R, wR, S | 0.0271, 0.0637, 1.03 | 0.0382, 0.1529, 1.17 | 0.0420, 0.1419, 1.15 | 0.0457, 0.1233, 1.13 | 0.0340, 0.0674, 0.99 | 0.0240, 0.0980, 1.18 |
4.4. Biological tests
Estrogenic activity was evaluated according to the already reported procedure.27a–c Thus, MCF-7 cells were plated in 1 mL of DMEM medium without phenol red at a density of 3 × 104 cells. The following day, 1 mL of the same medium containing IC50 values of the 1–5 to be tested was added to the plates. After 48 h of incubation of 1–5, the medium was removed and fresh medium was added. After 5 days, the total protein content of the plate was analyzed by methylene blue staining. Cell monolayers were fixed for 1 h in methanol and afterwards the cells stained for 1 h with methylene blue (1 mg mL−1) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of methanol
1 mixture of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at 37 °C, then the cells washed thoroughly with water. Two mL of HCl (0.1 M) was then added and the absorbance of each well was measured at 620 nm with spectrophotometer.
water at 37 °C, then the cells washed thoroughly with water. Two mL of HCl (0.1 M) was then added and the absorbance of each well was measured at 620 nm with spectrophotometer.
4.5. Hirshfeld surface analysis
The volumes of Hirshfeld surface were calculated with CrystalExplorer (Version 3.1).354.6. Docking studies
The MolDock scoring function implemented in the Molegro Virtual Docker software (http://www.molegro.com)36 was used for the molecular docking procedure. The crystal structure 4-hydroxytamoxifen with the ER-α receptor was obtained from the Protein Data Bank (http://www.pdb.org, pdb ID: 3ERT). The docked derivatives were ranked according to the “reranking score” scheme which is a weighted linear combination of the intermolecular (steric, van der Waals, hydrogen bonding, electrostatic) between the ligand and the protein, and intramolecular interactions (torsional, sp2–sp2, steric, van der Waals, hydrogen bonding, electrostatic) of the ligand expressed in arbitrary units. Energy minimization of the docked structures was performed post docking. No geometric constraints were imposed to the structures obtained by X-ray diffraction.Acknowledgements
This research was carried out in partial fulfillment of the requirements for the master thesis of M. A. under the supervision of I. I. O. I. I. O. and M. A. acknowledge the financial support from The Scientific and Technological Research Council of Turkey (TUBITAK, Project No. 113Z534). CNB and SKH would like to thank the Unit of bioactivity testing of xenobiotics, the University of Ioannina, for providing access to the facilities. CNB and SKH acknowledge the National Scholarships Foundation of Greece (IKY) for the post doctoral research fellowship of excellence programm IKY-Siemens.References
- H. Suziki and Y. Matano, Organobismuth Chemistry, Elsevier Science, 2001 Search PubMed.
- T. Yagura, Biological Activity of Organobismuth Compounds, Res. Adv. Antimicrob. Chemother., 2006, 6, 19 Search PubMed.
- H. Sun, H. Li and J. Sadler, Chem. Ber., 1997, 130, 669 CrossRef CAS.
- N. Yang and H. Sun, Coord. Chem. Rev., 2007, 251, 2354 CrossRef CAS.
- E. R. T. Tiekink, Crit. Rev. Oncol. Hematol., 2002, 42, 217 CrossRef PubMed.
- R. Huang, A. Wallqvist and D. G. Covell, Biochem. Pharmacol., 2005, 69, 1009 CrossRef CAS PubMed.
- S. M. Skinner, J. M. Swatzell and R. W. Lewis, Res. Commun. Chem. Pathol. Pharmacol., 1978, 17, 165 Search PubMed.
- S. Kirschner, Y. Wei, D. Francis and J. G. Bergman, J. Med. Chem., 1966, 9, 369 CrossRef CAS PubMed.
- Anticancer activity of molecular compounds of arsenic, antimony and bismuth, Biological Chemistry of Arsenic, Antimony and Bismuth, ed. E. R. T. Tiekink and H. Sun, John Wiley & Sons, New York, 2011, pp. 293–310 Search PubMed.
- (a) W. Friebolin, G. Schilling, M. Zoller and E. Amtmann, J. Med. Chem., 2005, 48, 7925 CrossRef CAS PubMed; (b) H. Li, C. S. Lai, J. Wu, P. C. Ho, D. de Vos and E. R. T. Tiekink, J. Inorg. Biochem., 2007, 101, 809 CrossRef CAS PubMed; (c) D. H. A. Ishak, K. K. Ooi, K. P. Ang, A. M. Akim, Y. K. Cheah, N. Nordin, S. N. B. A. Halim, H. L. Seng and E. R. T. Tiekink, J. Inorg. Biochem., 2014, 130, 38–51 CrossRef CAS PubMed; (d) M. Li, Y. Lu, M. Yang, Y. Li, L. Zhang and S. Xie, Dalton Trans., 2012, 41, 12882–12887 RSC; (e) M.-X. Li, M. Yang, J.-Y. Niu, L.-Z. Zhang and S.-Q. Xie, Inorg. Chem., 2012, 51, 12521–12526 CrossRef CAS PubMed; (f) N. Zhang, Y. Tai, M. Li, P. Ma, J. Zhao and J. Niu, Dalton Trans., 2014, 43, 5182–5189 RSC.
- I. I. Ozturk, C. N. Banti, N. Kourkoumelis, M. J. Manos, A. J. Tasiopoulos, A. M. Owczarzak, M. Kubicki and S. K. Hadjikakou, Polyhedron, 2014, 67, 89 CrossRef CAS.
- G. D. Thorn and R. Ludwig, The Dithiocarbamates and Related Compounds, Elsevier, Amsterdam, 1981 Search PubMed.
- (a) E. J. Butterfield and D. C. Torgeson, Fungicides, in Kirk-Ortmer Encyclopedia of Chemical Technology, ed. H. F. Mark, D. F. Othmer, C. G. Overberger and G. T. Seaborg, Wiley, New York, 1991 Search PubMed; (b) P. K. Gessner and T. Gessner, Disulfiram and its Metabolite, Diethydithiocarbamate, Chapman and Hall, London, 1992 Search PubMed; (c) Y. Tatara, Y. C. Lin, Y. Bamba, T. Mori and T. Uchida, Biochem. Biophys. Res. Commun., 2009, 384, 394 CrossRef CAS PubMed.
- L. I. Viktoriano, Coord. Chem. Rev., 2000, 196, 383 CrossRef.
- (a) J. A. McCleverty and N. Morrison, J. Chem. Soc., Dalton Trans., 1976, 21, 2169 RSC; (b) B. A. Prakasam, K. Ramalingam, G. Bocelli and A. Cantoni, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2020 CrossRef CAS; (c) L. I. Victoriano, Polyhedron, 2000, 19, 2269 CrossRef CAS.
- (a) P. T. Beurskens, W. P. Bosman and J. A. Cras, J. Cryst. Mol. Struct., 1972, 2, 183 CrossRef CAS; (b) J. Willemse and J. J. Steggerda, J. Chem. Soc. D, 1969, 19, 1123 RSC.
- (a) H. Abrahamson, J. R. Heiman and L. H. Pignolet, Inorg. Chem., 1975, 14, 2070 CrossRef CAS; (b) E. R. T. Tiekink, X. F. Yan and C. G. Young, Aust. J. Chem., 1992, 45, 897 CrossRef CAS; (c) R. N. Jowitt and P. C. H. Mitchell, Inorg. Nucl. Chem. Lett., 1968, 4, 39 CrossRef CAS; (d) Z. B. Varadi and A. Nieuwpoort, Inorg. Nucl. Chem. Lett., 1974, 10, 801 CrossRef CAS; (e) A. Nieuwpoort, H. M. Claessen and J. G. M. van de Linden, Inorg. Nucl. Chem. Lett., 1975, 11, 869 CrossRef CAS; (f) P. J. H. A. M. van de Leemput, J. A. Cras and J. Willemse, Recl. Trav. Chim. Pays-Bas, 1977, 96, 78 Search PubMed.
- (a) R.-Z. Sun, Y.-C. Guo and W.-M. Liu, Chin. J. Struct. Chem., 2012, 31, 655 CAS; (b) H. P. S. Chauhan, A. Bakshi and S. Bhatiya, Spectrochim. Acta, Part A, 2011, 81, 417 CrossRef CAS PubMed; (c) H. P. S. Chauhan, A. Bakshi and S. Bhatiya, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 345 CrossRef CAS; (d) C. L. Teske and W. Bensch, Z. Anorg. Allg. Chem., 2011, 637, 406 CrossRef CAS; (e) H. P. S. Chauhan, Appl. Organomet. Chem., 2010, 24, 317 CAS; (f) H. P. S. Chauhan and U. P. Singh, Appl. Organomet. Chem., 2007, 21, 880 CrossRef CAS; (g) H. Yin, F. Li and D. Wang, J. Coord. Chem., 2007, 60, 1133 CrossRef CAS; (h) R. W. Gable, B. F. Hoskins, R. J. Steen, E. D. R. Tiekink and G. Winter, Inorg. Chim. Acta, 1983, 74, 15 CrossRef CAS.
- (a) H. P. S. Chauhan, N. M. Shaik and U. P. Singh, Appl. Organomet. Chem., 2006, 20, 142 CrossRef CAS; (b) H. P. S. Chauhan and U. P. Singh, Appl. Organomet. Chem., 2006, 20, 404 CrossRef CAS; (c) H. P. S. Chauhan, K. Kori, N.-M. Shaik, S. Mathur and V. Huch, Polyhedron, 2005, 24, 89 CrossRef CAS; (d) Y. Liu and E. R. T. Tiekink, Appl. Organomet. Chem., 2004, 18, 299 CrossRef CAS; (e) H.-D. Yin and C.-H. Wang, Appl. Organomet. Chem., 2004, 18, 420 CrossRef CAS; (f) H.-D. Yin and C.-H. Wang, Appl. Organomet. Chem., 2004, 18, 199 CrossRef CAS; (g) C. S. Lai and E. R. T. Tiekink, Appl. Organomet. Chem., 2003, 17, 195 CrossRef CAS; (h) S. S. Garje and V. K. Jain, Coord. Chem. Rev., 2003, 236, 35 CrossRef CAS; (i) I. Baba, S. Ibrahim, Y. Farina, A. H. Othman, I. A. Razak, H.-K. Fun and S. W. Ng, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2001, 57, m39 CAS; (j) V. Venkatachalam, K. Ramalingam, G. Bocelli and A. Cantoni, Inorg. Chim. Acta, 1997, 261, 23 CrossRef CAS.
- (a) C. L. Raston, G. L. Rowbottom and A. H. White, J. Chem. Soc., Dalton Trans., 1981, 6, 1372 RSC; (b) C. L. Raston, G. L. Rowbottom and A. H. White, J. Chem. Soc., Dalton Trans., 1981, 6, 1369 RSC; (c) C. L. Raston, G. L. Rowbottom and A. H. White, J. Chem. Soc., Dalton Trans., 1981, 6, 1383 RSC; (d) C. L. Raston, G. L. Rowbottom and A. H. White, J. Chem. Soc., Dalton Trans., 1981, 6, 1352 RSC; (e) C. L. Raston, G. L. Rowbottom and A. H. White, J. Chem. Soc., Dalton Trans., 1981, 6, 1379 RSC.
- (a) S. K. Hadjikakou, C. D. Antoniadis, N. Hadjiliadis, M. Kubicki, J. Binolis, S. Karkabounas and K. Charalabopoulos, Inorg. Chim. Acta, 2005, 358, 2861 CrossRef CAS; (b) I. I. Ozturk, S. K. Hadjikakou, N. Hadjiliadis, N. Kourkoumelis, M. Kubicki, M. Baril, I. S. Butler and J. Balzarini, Inorg. Chem., 2007, 46, 8652 CrossRef CAS PubMed; (c) I. I. Ozturk, S. K. Hadjikakou, N. Hadjiliadis, N. Kourkoumelis, M. Kubicki, A. J. Tasiopoulos, H. Scleiman, M. M. Barsan and I. S. Butler, Inorg. Chem., 2009, 48, 2233 CrossRef CAS PubMed; (d) S. K. Hadjikakou, I. I. Ozturk, M. N. Xanthopoulou, P. C. Zachariadis, S. Zartilas, S. Karkabounas and N. Hadjiliadis, J. Inorg. Biochem., 2008, 102, 1007 CrossRef CAS PubMed; (e) I. I. Ozturk, S. Filimonova, S. K. Hadjikakou, N. Kourkoumelis, V. Dokorou, M. J. Manos, A. J. Tasiopoulos, M. M. Barsan, I. S. Butler, E. R. Milaeva, J. Balzarini and N. Hadjiliadis, Inorg. Chem., 2010, 49, 488 CrossRef CAS PubMed; (f) I. I. Ozturk, A. K. Metsios, S. Filimonova-Orlova, N. Kourkoumelis, S. K. Hadjikakou, E. Manos, A. J. Tasiopoulos, S. Karkabounas, E. R. Milaeva and N. Hadjiliadis, Med. Chem. Res., 2012, 21, 3523 CrossRef CAS; (g) I. I. Ozturk, N. Kourkoumelis, S. K. Hadjikakou, M. J. Manos, A. J. Tasiopoulos, I. S. Butler, J. Balzarini and N. Hadjiliadis, J. Coord. Chem., 2011, 64, 3859 CrossRef CAS; (h) I. I. Ozturk, C. N. Banti, M. J. Manos, A. J. Tasiopoulos, N. Kourkoumelis, K. Charalabopoulos and S. K. Hadjikakou, J. Inorg. Biochem., 2012, 109, 57 CrossRef CAS PubMed; (i) I. I. Ozturk, O. S. Urgut, C. N. Banti, N. Kourkoumelis, A. M. Owczarzak, M. Kubicki, K. Charalabopoulos and S. K. Hadjikakou, Polyhedron, 2013, 52, 1403 CrossRef CAS; (j) I. I. Ozturk, O. S. Urgut, C. N. Banti, N. Kourkoumelis, A. M. Owczarzak, M. Kubicki and S. K. Hadjikakou, Polyhedron, 2014, 70, 172 CrossRef CAS; (k) A. Han, I. I. Ozturk, C. N. Banti, N. Kourkoumelis, M. Manoli, A. J. Tasiopoulos, A. M. Owczarzak, M. Kubicki and S. K. Hadjikakou, Polyhedron, 2014, 79, 151 CrossRef CAS.
- H. P. S. Chauhan and U. P. Singh, Appl. Organomet. Chem., 2006, 20, 404 CrossRef CAS.
- (a) M. M. Coleman, J. L. Koenig and J. R. Shelton, J. Polym. Sci., 1974, 12, 1001 CAS; (b) M. M. Milosavljevic, A. D. Morinkovic, J. M. Morkovic, D. V. Brkovic and M. M. Milosavljevic, Chem. Ind. Chem. Eng. Q., 2012, 18, 73 CrossRef CAS.
- (a) F. Zouari and A. B. Salah, Phase Transitions, 2005, 78, 317 CrossRef CAS; (b) S. R. Luan, Y. H. Zhu, Y. Q. Jia and Q. Cao, J. Therm. Anal. Calorim., 2010, 99, 523 CrossRef CAS; (c) J. Laane and P. W. Jagodzinski, Inorg. Chem., 1980, 19, 44 CrossRef CAS; (d) K. Ichikawa and K. Fukushi, J. Raman Spectrosc., 1986, 17, 139 CrossRef CAS; (e) R. P. Oertel and R. A. Plane, Inorg. Chem., 1967, 6, 1960 CrossRef CAS.
- S. S. Batsanov, Inorg. Mater., 2001, 37, 871 CrossRef CAS translated from; Neorg. Mater. 2001, 37, 1031.
- (a) Y. Wang, J. H. Liao and C. H. Ueng, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1986, 42, 1420 CrossRef; (b) M. Colapietro, A. Domenicano and A. Vaciago, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1976, 32, 2581 CrossRef.
- (a) S. Clede, F. Lambert, C. Sandt, S. Kascakova, M. Unger, E. Harte, M. Plamont, R. Saint-Fort, A. Deniset-Besseau, Z. Gueroui, C. Hirschmugl, S. Lecomte, A. Dazzi, A. Vessieres and C. Policar, Analyst, 2013, 138, 5627–5638 RSC; (b) A. Vessieres, S. Top, P. Pigeon, E. Hillard, L. Boubeker, D. Spera and G. Jaouen, J. Med. Chem., 2005, 48, 3937–3940 CrossRef CAS PubMed; (c) D. Plazuk, S. Top, A. Vessieres, M. Plamont, M. Huche, J. Zakrzewski, A. Makal, K. Wozniak and G. Jaouen, Dalton Trans., 2010, 39, 7444–7450 RSC; (d) S. Kim-Schulze, K. A. McGowan, S. C. Hubchak, M. C. Cid, M. Beth Martin, H. K. Kleinman, G. L. Greene and H. William Schnaper, Circulation, 1996, 94, 1402 CrossRef CAS PubMed; (e) J. I. Hirsch, W. L. Banks, J. S. Sullivan and J. S. Horsley, Radiology, 1989, 105–107 CrossRef CAS PubMed; (f) M. Oz, D. E. Lorke, M. Hasan and G. A. Petroianu, Med. Res. Rev., 2011, 31, 93–117 CrossRef CAS PubMed; (g) Y. Potier, S. J. Elliot, I. Tack, O. Lenz, G. E. Striker, L. J. Striker and M. Karl, J. Am. Soc. Nephrol., 2001, 12, 241–251 Search PubMed; (h) Y. Berthois, J. A. Katzenellenbogent and B. S. Katzenellenbogen, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 2496–2500 CrossRef CAS PubMed.
- (a) A. Núñez-Montenegro, R. Carballo and E. M. Vázquez-López, J. Inorg. Biochem., 2014, 140, 53–63 CrossRef PubMed; (b) R. Kumar, M. N. Zakharov, S. H. Khan, R. Miki, H. Jang, G. Toraldo, R. Singh, S. Bhasin and R. Jasuja, J. Amino Acids, 2011, 812540, DOI:10.4061/2011/812540; (c) A. Strom, J. Hartman, J. S. Foster, S. Kietz, J. Wimalasena and J. A. Gustafsson, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1566–1571 CrossRef PubMed; (d) G. Lazennec, D. Bresson, A. Lucas, C. Chauveau and F. Vignon, Endocrinology, 2001, 142, 4120–4130 CAS; (e) K. R. A. Abdellatif, A. Belal and H. A. Omar, Bioorg. Med. Chem. Lett., 2013, 23, 4960–4963 CrossRef CAS PubMed; (f) A. K. Shiau, D. Barstad, P. M. Loria, L. Cheng, P. J. Kushner, D. A. Agard and G. L. Greene, Cell, 1998, 95, 927–937 CrossRef CAS PubMed; (g) A. M. Brzozowski, A. C. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson and M. Carlquist, Nature, 1997, 389, 753 CrossRef CAS PubMed; (h) A. Nguyen, S. Top, A. Vessières, P. Pigeon, M. Huché, E. A. Hillard and G. Jaouen, J. Organomet. Chem., 2007, 692, 1219–1225 CrossRef CAS; (i) L. Zheng, Q. Wei, B. Zhou, L. Yang and Z. L. Liu, Anti-Cancer Drugs, 2007, 18, 1039–1044 CrossRef CAS PubMed.
- O. S. Urgut, I. I. Ozturk, C. N. Banti, N. Kourkoumelis, M. Manoli, A. J. Tasiopoulos and S. K. Hadjikakou, Mater. Sci. Eng., C, 2016, 58, 396–408 CrossRef CAS PubMed.
- (a) T. Cheeseright, M. Mackey, S. Rose and J. G. Vinter, Expert Opin. Drug Discovery, 2007, 2, 131–144 CrossRef CAS PubMed; (b) T. Cheeseright, M. Mackey, S. Rose and J. G. Vinter, J. Chem. Inf. Model., 2006, 46, 665–676 CrossRef CAS PubMed; (c) D. Rogers and M. Hahn, J. Chem. Inf. Model., 2010, 50, 742–754 CrossRef CAS PubMed; (d) M. Hassan, R. D. Brown, S. Varma-O'brien and D. Rogers, Mol. Diversity, 2006, 10, 283–299 CrossRef CAS PubMed.
- CrysAlis RED, version 1.171.31.5, Oxford Diffraction Ltd., 2006, release 28-08-2006 CrysAlis171.NET Search PubMed.
- Oxford Diffraction, Crysalis CCD and Crysalis RED, Version p171.29.2, Oxford Diffraction Ltd, Abingdon, Oxford, England, 2006 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467 CrossRef.
- G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, CrystalExplorer (Version 3.1), University of Western Australia, 2012 Search PubMed.
- R. Thomsen and M. H. Christensen, J. Med. Chem., 2006, 49, 3315–3321 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data for complexes 1–5. CCDC 1445039 (1a), 1445037 (1b), 1445036 (2), 1445041 (3), 1445040 (4) and 1445038 (5). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra01181k |
| This journal is © The Royal Society of Chemistry 2016 |