Porous β-MnO2 nanoplates derived from MnCO3 nanoplates as highly efficient electrocatalysts toward oxygen evolution reaction†
Abstract
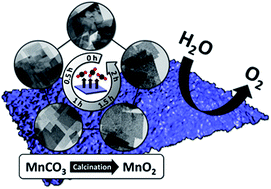
β-MnO2 has not been considered as an effective catalyst toward the oxygen evolution reaction due to its lack of active di-μ2-oxo bridged Mn centres and inaccessibility to the inner Mn atoms. We have envisioned that β-MnO2 can be made catalytically active by making the inner Mn atoms accessible. In order to accomplish this, we have synthesized MnCO3 nanoplates via a solution route and converted them into highly porous β-MnO2 nanoplates with very high surface area. In addition to the reduced overpotential of 450 mV at 10 mA cm−2, the derived Tafel slope was 78.2 mV dec−1, showing a superior catalytic activity of the porous nanoplate, which is comparable to the catalytic performance of best performing α-MnO2 phase. The importance of surface-bound catalytic Mn sites in highly porous β-MnO2 nanoplates is also demonstrated by Au loading-induced blockage of them and corresponding catalytic activity deterioration.

 Please wait while we load your content...
Please wait while we load your content...