Synthesis of well-defined alkyne terminated poly(N-vinyl caprolactam) with stringent control over the LCST by RAFT†
Abstract
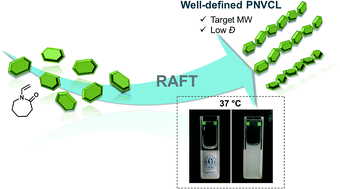
The reversible addition–fragmentation chain transfer (RAFT) of N-vinyl caprolactam (NVCL) using two new xanthates with alkyne functionalities is reported. The kinetic data obtained for polymerization of this non-activated monomer using a protected alkyne-terminated RAFT agent (PAT-X1) revealed a linear increase of the polymer molecular weight with the monomer conversion as well as low dispersity (Đ) during the entire course of the polymerization. The system reported here allowed us to enhance the final conversion, diminish Đ and reduce the polymerization temperature compared to the typical values reported in the scarce literature available for the RAFT polymerization of NVCL. The resulting PNVCL was fully characterized using 1H nuclear magnetic resonance (1H NMR), matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS), Fourier-transform infrared spectroscopy (FTIR) and gel permeation chromatography (GPC) techniques. The temperature-responsive features of PNVCL in aqueous solutions were fully investigated under different conditions using turbidimetry. The presented strategy allows the synthesis of well-defined PNVCL with sharp and reversible phase transition temperatures around 37 °C. By manipulating the polymer molecular weight, or the solution properties, it is possible to tune the PNVCL phase transition. As a proof-of concept, the alkyne functionalized PNVCL was used to afford new linear block copolymers, by reacting with an azide-terminated poly(ethylene glycol) (N3-PEG) through the copper catalyzed azide–alkyne [3 + 2] dipolar cycloaddition (CuAAC) reaction. The results presented establish a robust system to afford the synthesis of PNCVL with fine tuned characteristics that will enable more efficient exploration of the remarkable potential of this polymer in biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...