Catalytic dehydration of 2,3-butanediol over P/HZSM-5: effect of catalyst, reaction temperature and reactant configuration on rearrangement products
Jinbo Zhaoa,
Dinghua Yu*ab,
Wengui Zhanga,
Yi Huab,
Ting Jianga,
Jie Fuc and
He Huang*ab
aCollege of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. E-mail: yudh@njtech.edu.cn; biotech@njtech.edu.cn
bState Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, 5 Xinmofan Road, Nanjing 210009, China
cKey Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, 38 Zheda Road, Hangzhou 310027, China
First published on 26th January 2016
Abstract
As a type of important bio-based vicinal diol, 2,3-butanediol could be transformed into methyl ethyl ketone and 2-methyl propanal through a pinacol rearrangement mechanism under acid catalysis conditions. In this paper, a series of P/HZSM-5 (Si/Al = 360) samples with various phosphate contents were prepared and tested via the catalytic transformation of 2,3-butanediol, with particular focus on the effect of phosphate content on the ratio of methyl ethyl ketone to 2-methyl propanal. The catalyst structures were studied using several physico-chemical methods such as XRD, N2 sorption, NH3-TPD and FT-IR. At 180 °C, the ratio of methyl ethyl ketone to 2-methyl propanal increased from 5.1 to 37.5 when the content of phosphate increased from 0.5 to 8.0. When the reaction temperature increased from 180 °C to 300 °C over 4% P2O5/HZSM-5, the ratio of methyl ethyl ketone to 2-methyl propanal decreased from 15.6 to 2.5. The configuration of 2,3-butanediol would affect the conversion but not the selectivity. The characterization results demonstrated that the phosphate modification of HZSM-5 could not only reduce the strong and medium acid sites but also produce new weak acid sites. Strong acid sites and high reaction temperatures could promote the formation of 2-methyl propanal through methyl migration via carboniums. Based on these results, a possible surface reaction model was proposed.
1. Introduction
For the sustainable chemical industry, biomass resources could be used as renewable feedstock to produce chemicals and fuels and thus reduce the reliance on fossil resources.1–3 Considerable efforts are currently being invested in biotechnology4 and green chemistry to develop a sustainable chemical industry using bio-renewable sources. Although the biological fermentation process could transform bio-based carbohydrates into bulky platform chemicals,5 such as polyol, organic acids and amino acids, the substrate specificity would limit the biological synthesis path diversity to the acquisition of various chemicals. Compared with the biological process, chemical catalysis could provide a versatile protocol for bio-based chemical derivatization.6 Many studies have been devoted to developing chemical catalysis routes for the conversion of platform chemicals such as lactic acid,7 sorbitol8 and ketoses (fructose, sorbose, tagatose).9 Excessive hydroxyl groups in polyols could particularly induce the complex reaction chemistry, which should be studied for the development of efficient biorenewable catalysis processes.2,3-Butanediol, a typical platform chemical, was produced from a carbohydrate through biological fermentation.10 Through chemical catalytic transformation, as shown in Scheme 1, 2,3-butanediol could be transformed into diversified products that could find important applications in many fields such as solvents, cosmetics, plasticizers, foods, moistening and softening agents, and pharmaceuticals. For example, 2,3-butanediol could be dehydrated11–13 into methyl ethyl ketone, 2-methyl propanal, 3-buten-2-ol and 1,3-butadiene, oxidized into 3-hydroxyl-butanone and diacetyl,14 or polymerized with diacid or isocyanate to produce polymers such as polyester15 or polyurea.16 These derivatives could be acquired through hydroxyl reaction chemistry.
 | ||
| Scheme 1 Hydroxyl reaction chemistry of 2,3-butanediol and the corresponding catalytic mechanism and product distribution. | ||
Recently, many studies have been devoted to the development of heterogeneous catalysts for 2,3-butanediol. According to the results reported by Toeroek et al.,17 diol could undergo pinacol rearrangement to produce the corresponding carbonyl compounds, and its selectivity is dependent on its acid strength and the form of the catalysts. Different zeolites18–20 including X, Y, modernite, β and ZSM-5 have been used for 2,3-butanediol dehydration, and the product of methyl ethyl ketone formation was favored. These catalysts are focused on zeolites with a low Si/Al ratio, and the reaction was proceeded at a high temperature (230–350 °C). Under acid catalysis conditions, 2,3-butanediol could be transformed into methyl ethyl ketone (MEK), 2-methyl propanal (MPA), or 1,3-butadiene. Therefore, the reaction network adjustment would be expected through the selection of a suitable catalyst. In our previous report, MEK and MPA could be produced from 2,3-BDL with HZSM-5 zeolites with different Si/Al ratios. Therefore, acid catalytic sites of HZSM-5 could not only promote dehydration to MEK but also catalyze the rearrangement of carbonium to MPA. Few studies have investigated the reaction balance of dehydration and the rearrangement process and the reaction network adjustment through catalyst modification and reaction parameters. In total, even less attention has been paid to the effect of the typical molecular structure, vicinal hydroxyls, and their configuration on catalytic performance, which is a common feature of bio-based chemical transformation.
The aim of the present contribution is to investigate the effect of catalyst structure and reaction temperature on product distribution in rearrangement via carbonium during 2,3-butanediol catalytic dehydration. Phosphate-modified HZSM-5 zeolites could improve the hydrothermal stabilization during the alkylene crack process,21 and the surface structure model of phosphate-modified HZSM-5 has been proposed.21–23 In this paper, a series of HZSM-5 (Si/Al = 360) catalysts with different phosphate content were prepared and used to study the influence of catalyst structure on the pinacol rearrangement process. The pore structures and surface acidity were elucidated by several physico-chemical methods such as XRD, N2 sorption, FT-IR and NH3-TPD. Based on this structural information, a possible surface reaction mechanism was proposed. These results would be useful in catalyst design and in the reaction chemistry of bio-based chemical conversion.
2. Experimental section
2.1 Catalyst preparation
HZSM-5 zeolites (Si/Al = 360) were obtained from the Catalyst Factory of Nankai University. Phosphate-modified zeolites were prepared by incipient wetness impregnation, as follows: the mixture of 10 g HZSM-5(360) in 40 mL of deionized water was stirred for 30 min. The specified amount of NH4H2PO4 was added into the mixture of zeolites to yield the desired content of P2O5 (0.5 wt%, 1.0 wt%, 2.0 wt%, 4.0 wt%, 6.0 wt% and 8.0 wt%). The impregnation process was conducted for 24 h at room temperature. The mixtures were then dried by rotary evaporators at 120 °C for 4 h, heated from 120 °C to 550 °C at a heating rate of 10 °C min−1 and calcined at 550 °C for 4 h. The samples were cooled to room temperature under ambient conditions and then tableted and crushed into 30–40 meshes, named “content%P/HZSM-5(360)”.2.2 2,3-Butanediol feedstock and distillation
Unless otherwise specified, the reactant feedstock was the 2,3-butanediol extract from fermentation broth, and the mixture constitution was determined as follows: 78.8% meso-2,3-butanediol and 21.2% racemic-2,3-butanediol. To study the effect of chiral configuration, the chiral ratio of reactant was changed via reduced pressure distillation. The separation condition was as follows: the stirring velocity was 20 rpm, the temperature was 110 °C, the vacuum degree was 29.3 mmHg, and the distillation temperature was 73 °C. After the distillation, the distillation residue was collected and used as a reactant, which comprises 80.5% meso-2,3-butanediol and 19.5% 2,3-butanediol of racemic isomers.2.3 Catalytic reaction and product determination
The catalytic reaction operation was performed similarly to the method reported in our group's previous study.11 Simply, catalytic dehydration was performed in a fixed-bed quartz reactor with an 8 mm inner diameter. The catalysts (1.5 g) with 30–40 meshes were charged in the middle section of the reactor, with quartz wool packed in both ends. Before catalytic evaluation, the catalyst was pretreated at the required reaction temperature for 0.5 h under highly pure N2 (0.1 MPa, 12.5 mL min−1). The feedstock, an aqueous solution of 2,3-butanediol (60 wt%), was then pumped into the preheating zone (input speed = 6 mL h−1), driven through the catalyst bed by nitrogen (0.1 MPa, 12.5 mL min−1). Unless otherwise specified, the reactant contains 78.8% meso-2,3-butanediol and 21.2% racemic-2,3-butanediol.Product analysis was carried out offline by a Shimadzu GC-2010 gas chromatograph using a TCD detector equipped with a Satbilwax-DA capillary column (30 m, ID 0.32 mm, film thickness 0.25 μm). The injector and detector temperatures were 280 and 300 °C, respectively. The oven was kept at 70 °C for 2 min then raised to 170 °C at a rate of 10 °C min−1, where it remained for 2 min before finally being raised to 215 °C at 15 °C min−1 for 2 min. For quantitative analysis, ethanol was selected as the internal standard. The corresponding relative correction factors of 2,3-butanediol, water, methyl ethyl ketone, 2-methyl propanal, and butadiene were 1.2249, 0.7024, 1.1934, 1.1585 and 1.0488, respectively.
2.4 Catalyst structure characterization
X-ray diffraction was performed on a Thermo ARL X'TRA X-ray diffractometer from Thermo Electron Corporation using Cu Kα radiation. The wavelength was 0.15406 nm. With a scanning speed of 5° min−1, the diffraction data were collected at 25 °C in a 2θ range from 5° to 75°.N2 sorption measurements were carried out on an ASAP2020 instrument from Micromeritics. Before the measurements, the catalysts (0.1000 g) were degassed under vacuum for 10 h at 300 °C. The specific surface area and micropore volume were calculated by the Brunauer–Emmett–Teller (BET) method and t-plot method, respectively. The micropore width distribution was acquired according to the Horvath–Kawazoe method.
The temperature-programmed desorption of ammonia was performed with a BEL-CAT-B-82 instrument connected to a thermal conductivity detector. The catalyst surfaces were cleaned by a flow of helium at 550 °C for 60 min. The samples were then cooled to 120 °C, and the ammonia was adsorbed at 120 °C for 60 min. The adsorbed samples were flushed with the flow of helium at 120 °C for 40 min. The TPD of ammonia was performed at a helium flow of 40 mL min−1 and a heating rate of 10 °C min−1 from 120–750 °C. The acid amount was determined by peak area acquired by integrating the TPD desorption peaks in the temperature range.
FT-IR (Fourier transform infrared spectroscopy) was carried out on an Avatar 360 FT-IR spectrometer from Nicolet using DTGS KBr as the detector and KBr as the beam splitter. The catalyst was mixed together with KBr powder and tableted into a thin round piece. The piece was then scanned in the range of 400–4000 cm−1.
3. Results and discussion
3.1 Catalytic dehydration performance of 2,3-butanediol
The modified HZSM-5 catalysts with different amounts of phosphates were tested by the catalytic dehydration of 2,3-butanediol at 180 °C, and the corresponding results are shown in Fig. 1. Compared with the parent HZSM-5 (Si/Al = 360), the conversion of 2,3-BDL over the modified zeolites (P2O5 content ≤ 2 wt%) improved from 32.7% to more than 90%, which indicates that these modified zeolites could be more efficient catalysts for 2,3-butanediol conversion than the parent zeolites. When the loaded amount of P2O5 was above 2 wt%, the modified zeolites exhibited very low conversions of 2,3-BDL even less than 15% over 6.0P/HZ. At meanwhile, the 2,3-BDL conversions over the modified zeolites ((P2O5 content ≤ 2 wt%) were all above 90%. This decrease might be attributed to the excessive P2O5 species over the zeolite surface, which led to the change of surface acidic properties. With the acid catalyst, 2,3-butanediol could undergo a dehydration reaction via a pinacol rearrangement mechanism.24 Over the HZSM-5 zeolite surface, methyl ethyl ketone is the main product of catalytic dehydration of 2,3-butanediol. During pinacol rearrangement, the products of methyl ethyl ketone and 2-methyl propanal come from hydride migration and methyl migration, respectively.25 As shown in Fig. 1, the ratio of methyl ethyl ketone to 2-methyl propanal (MEK/MPA) increased from 5 to 37.5 when the HZSM-5 was modified by phosphate via the P2O5 content increase from 0.5% to 8.0%. These product distribution change indicated that the catalyst modification could produce different surface structures and could further affect carbonium rearrangement kinetics and finally lead to different MEK/MPA ratios. The surface structure differences between modified catalysts should be elucidated via physico-chemical methods.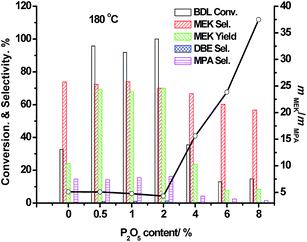 | ||
| Fig. 1 Catalytic results of 2,3-butanediol dehydration at 180 °C over P/HZSM-5(360) zeolites with various P2O5 contents. | ||
Undoubtedly, the reaction temperature would not only influence conversion but also affect product distribution because of the thermal and kinetic dynamics.26 Over HZSM-5 zeolites with different P2O5 contents, the effects of reaction temperature on catalytic performance have demonstrated similar regularity. The temperature effects of 2% P/HZSM-5 and 4% P/HZSM-5 on the catalytic dehydration performance of 2,3-butanediol are shown in Fig. 2. For 4% P/HZSM-5 (Fig. 2b), when the reaction temperature was increased from 180 °C to 200 °C or even higher, the conversion of 2,3-butanediol was improved from 35.5% to 100%, which indicated that a higher temperature would be favorable to 2,3-butanediol conversion, in agreement with the thermal dynamics principle. When the reaction temperature was increased, the selectivity to methyl ethyl ketone was improved from 66.6% to 81.3% and then decreased to 74.7% at 300 °C. Meanwhile, the selectivity to 2-methyl propanal was gradually improved from 4.3% to 24.3%. Although the selectivity to both methyl ethyl ketone and 2-methyl propanal was improved with increasing reaction temperature, the ratio of MEK/MPA could further reflect the effect of reaction temperature on product distribution. As shown in Fig. 2b, the ratio of MEK/MPA decreased from 15.6 to 2.5 when the reaction temperature increased from 180 °C to 300 °C, which indicated that over HZSM-5 zeolites catalysts, the high reaction temperature would promote the methyl migration process to produce MPA. For 2% P/HZSM-5 (Fig. 2a), the influences of temperature on the 2,3-butanediol conversion and methyl ethyl ketone yield were not significant. With the rise of temperature, the selectivity to 2-methyl propanal increased, and the ratio of MEK/MPA decreased, similar with the reaction behaviors over 4% P/HZSM-5. Research of the temperature effect on conversion and selectivity would be beneficial to witness the complex interaction of protonation and carbonium rearrangement during the 2,3-butanediol dehydration process.
 | ||
| Fig. 2 The effect of reaction temperature on product distribution with phosphate-modified zeolites. (a) 2% P2O5/HZSM-5; (b) 4% P2O5/HZSM-5. | ||
To study the effect of 2,3-butanediol configuration on catalytic results, two types of reactant feedstock with different meso/racemic ratios (feed A: 72.7% meso (M) +27.3% racemic (R); feed B: 80.5% meso (M) +19.5% racemic (R)) have been used to test the catalytic performance over 4.0% P/HZSM-5 at 180 °C. The catalytic results are shown in Fig. 3. When feed B with high content of meso-type (80.5% meso +19.5% racemic) was used as a reactant, the conversion of 2,3-butanediol decreased from 35.5% to 22.1%, and the unreacted meso-type isomers increased from 49.2% to 64.9%, which indicated that the meso-type 2,3-butanediol reaction could be unfavorable over phosphate-modified HZSM-5 catalysts compared with racemic isomers. Meanwhile, the selectivity to methyl ethyl ketone and 2-methyl propanal showed no obvious change when the reactants changed from feed A to feed B. This is because 2,3-butanediol dehydration is the typical pinacol rearrangement process, and the product distribution would depend on the reaction conditions. To elucidate the difference in catalytic results, it is necessary to thoroughly comprehend the surface structure of modified HZSM-5 zeolites and the mechanism of pinacol rearrangement.
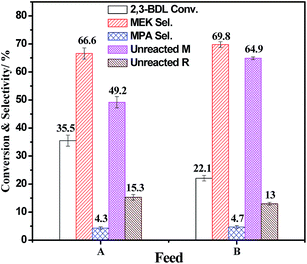 | ||
| Fig. 3 Catalytic performance over 4.0 P/HZ at 180 °C with different feed: (A) 72.7% meso (M) +27.3% racemic (R), (B) 80.5% meso (M) +19.5% racemic (R). | ||
3.2 Structure and acidity characterization
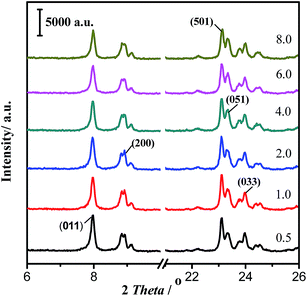
Zeolites are inorganic crystalline microporous materials, and the crystallization degree change could influence the catalytic performance. The effect of phosphate modification on the crystalline structure of HZSM-5 (Si/Al = 360) was studied, and the corresponding XRD patterns are shown in Fig. 4. All modified HZSM-5 samples with different P2O5 contents showed the characteristic diffraction peaks of ZSM-5 zeolites at 7.97°, 8.92°, 23.12°, 23.34°, 24°, which could be indexed into the typical ZSM-5 (JCPDS 44-0003) crystal planes (011), (200), (501), (051) and (033), respectively.27 With the increasing P2O5 content from 0.5% to 8.0%, the diffraction peak intensity gradually decreased. Moreover, compared with parent HZSM-5 (Si/Al = 360) zeolites, modified zeolite samples with phosphates show no new diffraction peaks, which suggest that no new crystalline phases or phosphate species could exist in the form of ions or highly dispersed clusters.To study the effect of phosphate modification on ZSM-5 pore structure, the parent and modified zeolite samples were tested using the N2 adsorption–desorption method, and the calculated results are shown in Table 1. As shown in Table 1, when the HZSM-5 sample was modified by 0.5% P2O5, the values of the specific surface area and micropore volume remained the same as those of the parent HZSM-5, which indicates that small amount phosphate modification has little influence on the pore structure of the catalysts. When the P2O5 content was increased from 1.0% to 8.0%, the specific surface area decreased from 325 m2 g−1 to 268 m2 g−1, and the micropore volume gradually decreased from 0.21 cm3 g−1 to 0.17 cm3 g−1. These surface and pore structure changes reflect the small destruction effect of phosphate modification on zeolite crystalline structures, which could be consistent with the results of XRD determination.
| Catalysts | BET surface areaa, (m2 g−1) | Volume, (cm3 g−1) | P2O5 contentsb, (wt%) |
|---|---|---|---|
| a The external surface area by t-plot method.b The actual phosphorus contents have been determined by XRF technique. | |||
| HZSM-5(360) | 325 | 0.21 | — |
| 0.5P/HZ | 327 | 0.21 | 0.42 |
| 1.0P/HZ | 316 | 0.20 | 0.85 |
| 2.0P/HZ | 308 | 0.19 | 1.81 |
| 4.0P/HZ | 288 | 0.18 | 3.75 |
| 6.0P/HZ | 275 | 0.18 | 5.22 |
| 8.0P/HZ | 268 | 0.17 | 7.35 |
On the surfaces of catalysts, the 2,3-butanediol molecules would undergo several steps, including the protonation of the hydroxyl group, water molecule elimination and carbonium formation and rearrangement. Therefore, the acidity of solid catalysts would influence the total reaction, including activity and selectivity with different products. To clarify the surface acidity properties, temperature-programmed desorption methods with NH3 probes (NH3-TPD) were used to study the surface acidity of parent and modified ZSM-5 catalysts. The desorption curves of ammonia are shown in Fig. 5. For the parent ZSM-5 (Si/Al = 360), the desorption curve of ammonia shows three desorption peaks, centered at 138 °C, 297 °C and 580 °C. The desorption temperature is higher; the acid intensity of active sites is stronger. Moreover, the ammonia desorption curve of parent ZSM-5 (Si/Al = 360) indicates that the parent catalyst has abundant strong acid sites. When the ZSM-5 (Si/Al = 360) zeolites were modified by different amounts of phosphates, the ammonia desorption curves showed the regular changes. With increasing P2O5 content, the desorption peaks at high temperatures became gradually weaker. Meanwhile, the desorption peaks at low and medium temperatures became gradually stronger. Moreover, the desorption peaks at low temperatures shifted to higher temperatures, as shown in Fig. 5. Notably, the samples with a P2O5 content higher than 2% showed significantly different ammonia desorption curves compared to those with a P2O5 content lower than 2%. The samples with a P2O5 content higher than 2% had obvious weak desorption peaks at high temperatures and strong desorption peaks at low temperatures. These changes in acidity properties could contribute to the different catalytic dehydration results of 2,3-butanediol.
From the qualitative results of acid intensity and amounts (Table 2), phosphate modification reduced the amount of strong acid sites from 1167 μmol g−1 over the parent HZSM-5 to 359 μmol g−1 over 8.0% P/HZSM-5 and increased the weak acid amount from 176 μmol g−1 over the parent HZSM-5 to 1093 μmol g−1 over 8.0% P/HZSM-5. However, the total acid amount showed no obvious change. Moreover, the desorption peak temperature corresponding to weak acid sites shifted from 138.5 °C over the parent HZSM-5 to 156.5 °C over 8.0% P/HZSM-5, which indicates that further phosphate addition would improve the weak acid intensity. These results demonstrate that phosphate modification adjusted the acid intensity distribution and maintained the total acid amount balance, and these inherent acidity properties directly influence the catalytic dehydration results of 2,3-butanediol, including the conversion of 2,3-butanediol and selectivity for MEK and MPA. Parent and modified HZSM-5 zeolites could be used as Brønsted type solid acids, whose active sites would mainly be produced by hydroxyl groups. FT-IR spectra could disclose the hydroxyl difference between the parent HZSM-5 and modified zeolites. Therefore, a series of modified HZSM-5 zeolites were studied by FT-IR, and the corresponding results are shown in Fig. 6.
| Catalysts | Desorption peak temperature/°C | Acid amount/μmol g−1 | Total acid amount/μmol g−1 | ||||
|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Weak | Medium | Strong | ||
| HZSM-5(360) | 138.5 | 297.0 | 580.9 | 176 | 315 | 1167 | 1658 |
| 0.5P/HZ | 138.4 | 289.5 | 578.9 | 208 | 329 | 1028 | 1565 |
| 1.0P/HZ | 150.1 | 306.2 | 587.9 | 306 | 328 | 878 | 1512 |
| 2.0P/HZ | 151.9 | 276.4 | 586.7 | 468 | 261 | 678 | 1407 |
| 4.0P/HZ | 161.0 | 343.7 | 576.6 | 893 | 201 | 360 | 1454 |
| 6.0P/HZ | 154.5 | 299.9 | 573.1 | 960 | 212 | 379 | 1551 |
| 8.0P/HZ | 156.5 | 288.2 | 579.3 | 1093 | 332 | 359 | 1784 |
From the transmission spectra show in Fig. 6a, the absorption peaks at 545, 802, 1064 and 1228 cm−1 could be attributed to the typical vibration of SiO4 tetrahedron units.28 According to the results reported by Armaroli, the absorption near 802 cm−1 could be induced by the symmetric stretching of the external linkages, and the absorption at 545 cm−1 is attributed to the double five-ring lattice vibration of the external linkages. The strong absorption between 1000 cm−1 and 1300 cm−1 could be assigned to the internal vibration of SiO4 or the AlO4 tetrahedra of ZSM-5. The absorption peak at 1064 cm−1 could be attributed to the asymmetric stretching vibration of the Si–O–T linkage. Compared with the parent HZSM-5, the absorption peak at 1064 cm−1 of modified HZSM-5 samples by phosphate showed a redshift, which indicates that the phosphate modification could affect the vibration of asymmetric stretching of Si–O–T linkage. Meanwhile, the absorption near 960 cm−1, the typical Si–O stretching vibration of the surface silanol groups,29 became gradually stronger when the zeolites were modified by increased phosphate amount. Moreover, the absorption near 960 cm−1 showed a redshift to a low wavenumber, which demonstrated that the phosphate modification could form a P–O–Si bond.
According to the results of Derouane et al.,30 the strong acidic sites on ZSM-5 zeolites could be produced by Al–OH located at the channel intersections, and the corresponding IR band and desorption peak are characterized by the peaks at 3600 cm−1 and 773 K, respectively. Weaker acidic sites are characterized by an IR band at 3720–3740 cm−1 and a desorption peak at approximately 500 K. They probably correspond to terminal silanol groups on the external surface of the zeolite or possibly to nonzeolitic impurities. Therefore, the acidity is stronger and the IR absorption wavenumber of the hydroxyl group is lower. Because ZSM-5 zeolites with high Si/Al ratio have stronger acidity, the IR absorption at 3450 cm−1, shown in Fig. 6(B), could be attributed to the strong acid sites. Meanwhile, the IR absorption peaks at 3650 cm−1 and 3744 cm−1 could be attributed to the medium and weak acidic sites. From the IR spectra of Fig. 6(B), the parent HZSM-5 had a strong absorption peak at 3450 cm−1, which could be attributed to strong acidic sites and should be produced by Al–OH. With the introduction of phosphate, the absorption peak at 3450 cm−1 became weak, and the absorption peak at 3650 cm−1 and 3744 cm−1 became strong, which is in agreement with the results of NH3-TPD. The absorption peaks at 3650 cm−1 and 3744 cm−1 could be attributed to the P–OH and Si–OH. In total, the absorption between 4000–3000 cm−1 is very weak, which demonstrates that the hydrophobic surface nature of HZSM-5(360) and small number of hydroxyl groups were produced by phosphate modification. This small amount of hydroxyl groups was also confirmed by the NH3-TPD results.
3.3 Surface reaction model of 2,3-butanediol over HZSM-5
2,3-Butanediol dehydration is a typical pinacol rearrangement, which can be observed when vicinal diols interact with acid. During the reaction process, the hydroxyl group is first protonated. When the water molecule is eliminated, the intermediate carbonium can form. Carbonium rearrangement through hydride shift or methyl shift can proceed selectively, depending on catalysts and reaction conditions. According to the classic organic chemistry principle, carbonium formation is the rate-determining step during the catalytic process of vicinal diols. When the carbonium formed, an alkyl group or hydrogen shifts over by one carbon towards a positive charge.To further clarify the 2,3-butanediol reaction process over modified HZSM-5, a possible surface acid sites structure and catalytic reaction model would be necessary. Regarding to the chemical states of phosphorus in ZSM-5 modified by phosphates, five possible models have been proposed.24b The catalysts preparation process would influence the possible chemical states and acid sites. In our research, the acid amount change and ammonia desorption curves showed that the number of OH groups had decreased after phosphate modification. Similar to the results reported by Ding et al.,24b these results have been ascribed to the condensation of two zeolitic hydroxyls and one phosphate molecule when the catalysts were calcined in dry air. Therefore, based on the FT-IR results and the literatures, the possible chemical states of phosphorus have been shown in Scheme 2.
Based on FT-IR and NH3-TPD results, the acid sites of modified HZSM-5 with phosphate could be produced by Al–OH and P–OH. 2,3-Butanediol protonation could be undertaken through adsorption at acidic sites such as Al–OH and P–OH. Meso-type 2,3-butanediol with two hydroxyl groups located on two sides of the C–C bond would prefer to interact with the acid sites through terminal adsorption (as shown in Scheme 2 state (I) and state (III)). Racemic type 2,3-butanediol with two hydroxyl groups on the same side of the C–C bond would produce terminal adsorption (state (IV)) and synergetic adsorption between two hydroxyls and phosphate active centers (state (III)). Because the acid intensity of Al–OH is stronger than that of P–OH, the terminal adsorption by Al–OH could efficiently activate the hydroxyl of 2,3-butanediol. In comparison, the acid intensity of P–OH is too weak to efficiently activate hydroxyl of 2,3-butanediol through terminal adsorption, but the bridge adsorption between phosphate and racemic isomers could compensate for the activation efficiency. Therefore, the protonation capability of acid sites could contribute to the conversion. These adsorption models could interpret the conversion differences induced by catalysts, reaction temperature and chiral configuration of the reactants.
After the H2O molecule was eliminated from the protonation state, the corresponding carbonium would form. The products of methyl ethyl ketone and 2-methyl propanal could come from hydride shift and methyl shift, respectively. According to the results reported by Alexander et al.,24a when treated with phosphoric acid, DL-2,3-butanediol could be converted into methyl ethyl ketone, whereas meso-2,3-butanediol could give a mixture of methyl ethyl ketone and 2-methyl propanal. From the product ratio of methyl ethyl ketone to 2-methyl propanal (MEK/MPA), the modified zeolites with higher phosphate content could acquire higher MEK/MPA ratios than those with lower phosphate content. Moreover, the increase in reaction temperature would reduce the MEK/MPA ratio. These results demonstrated that stronger acid sites and higher reaction temperatures would accelerate the methyl shift process. Moreover, the chiral configuration of 2,3-butanediol would have only the influence on the reactant-adsorbed state and carbonium formation. When the carbonium formed from any 2,3-butanediol isomers, the pinacol rearrangement product distribution would depend not on the reactant configuration but only on the catalysts and reaction temperature.
4. Conclusions
A series of phosphate-modified HZSM-5(360) catalysts have been prepared and used to catalyze 2,3-butanediol dehydration. The surface and porous structures have been disclosed by several physico-chemical methods including XRD, FT-IR, NH3-TPD and N2 sorption. The results showed that phosphate modification would reduce the surface strong acid sites while increasing weak and medium acid sites. The configuration of 2,3-butanediol would affect only the adsorption activation and carbonium formation process. Once the carbonium formed, the product distribution would be independent of the reactant configuration. Owing to the methyl shift being more difficult than the hydride shift during carbonium rearrangement, the strong acid and high reaction temperature would accelerate the methyl shift during the carbonium rearrangement process. These results would be useful for new catalysts and catalytic process design in bio-based chemical transformation, especially bio-based polyol with various configurations.Acknowledgements
The authors are grateful for the financial support by the National Natural Science Foundation of China (No. 21376120), the National Key Technology R&D Program (No. 2012BAD32B08), the National Basic Research Program (No. 2011CB710806), and the PhD Programs Foundation of Ministry of Education of China (No. 20113221110010).References
- (a) J. H. Clark, Nat. Chem., 2009, 1, 12–13 CrossRef CAS PubMed; (b) J. J. Bozell, Science, 2010, 329, 522–523 CrossRef CAS PubMed; (c) S. V. de Vyver, J. Geboers, P. A. Jacobs and B. F. Sels, ChemCatChem, 2011, 3, 82–94 CrossRef; (d) A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- T. P. Vispute, H. Zhang, A. Sanna, R. Xiao and G. W. Huber, Science, 2010, 330, 1222–1227 CrossRef CAS PubMed.
- J. Q. Bond, D. M. Alonso, D. Wang, R. M. West and J. A. Dumesic, Science, 2010, 327, 1110–1114 CrossRef CAS PubMed.
- B. Erickson, R. Singh and P. Winters, Science, 2011, 333, 1254–1256 CrossRef CAS PubMed.
- T. A. Werpy and G. Petersen, Top Value Added Chemicals from Biomass. Volume I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas, 2004 Search PubMed.
- (a) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed; (b) C. H. Zhou, J. N. Beltramini, Y. X. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC; (c) T. Schwartz, B. O'Neill, B. H. Shanks and J. A. Dumesic, ACS Catal., 2014, 4, 2060–2069 CrossRef CAS; (d) S. Dutta, S. De, B. Saha and M. I. Alam, Catal. Sci. Technol., 2012, 2, 2025–2036 RSC; (e) D. A. Simonetti and J. A. Dumesic, Catal. Rev.: Sci. Eng., 2009, 51, 441–484 CrossRef CAS.
- (a) P. Sun, D. Yu, Z. Tang, H. Li and H. Huang, Ind. Eng. Chem. Res., 2010, 49, 9082–9087 CrossRef CAS; (b) H. Wang, D. Yu, P. Sun, J. Yan, Y. Wang and H. Huang, Catal. Commun., 2008, 9, 1799–1803 CrossRef CAS; (c) P. Sun, D. Yu, K. Fu, M. Gu, Y. Wang, H. Huang and H. Ying, Catal. Commun., 2009, 10, 1345–1349 CrossRef CAS; (d) J. Peng, X. Li, C. Tang and W. Bai, Green Chem., 2014, 16, 108–111 RSC; (e) J. Zhang, Y. Zhao, X. Feng, M. Pan, J. Zhao, W. Ji and C. T. Au, Catal. Sci. Technol., 2014, 4, 1376–1385 RSC; (f) P. Mäki-Arvela, I. L. Simakova, T. Salmi and D. Y. Murzin, Chem. Rev., 2013, 114, 1909–1971 CrossRef PubMed.
- (a) J. Xia, D. Yu, Y. Hu, B. Zou, P. Sun, H. Li and H. Huang, Catal. Commun., 2011, 12, 544–547 CrossRef CAS; (b) M. Gu, D. Yu, H. Zhang, P. Sun and H. Huang, Catal. Lett., 2009, 133, 214–220 CrossRef CAS; (c) H. Li, D. Yu, Y. Hu, P. Sun, J. Xia and H. Huang, Carbon, 2010, 48, 4547–4555 CrossRef CAS; (d) J. Li, A. Spina, J. A. Moulijn and M. Makkee, Catal. Sci. Technol., 2013, 3, 1540–1546 RSC.
- (a) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC; (b) R. J. Putten, J. N. M. Soetedjo, E. A. Pidko, J. C. van der Waal, E. J. M. Hensen, E. de Jong and H. J. Heeres, ChemSusChem, 2013, 6, 1681–1687 CrossRef PubMed; (c) V. V. Ordomsky, J. van der Schaaf, J. C. Schouten and T. A. Nijhuis, ChemSusChem, 2012, 5, 1812–1819 CrossRef CAS PubMed; (d) C. Aellig and I. Hermans, ChemSusChem, 2012, 5, 1737–1742 CrossRef CAS PubMed; (e) H. Wang, Q. Kong, Y. Wang, T. Deng, C. Chen, X. Hou and Y. Zhu, ChemCatChem, 2014, 6, 728–732 CrossRef CAS.
- X. Ji, H. Huang and P. Ouyang, Biotechnol. Adv., 2011, 29, 351–364 CrossRef CAS PubMed.
- W. Zhang, D. Yu, X. Ji and H. Huang, Green Chem., 2012, 14, 3441–3450 RSC.
- H. Duan, D. Sun, Y. Yamada and S. Sato, Catal. Commun., 2014, 48, 1–4 CrossRef CAS.
- M. E. Winfield, J. C. S. I. R., 1945, 18, 412–423 CAS.
- O. A. Anunziata, L. B. Pierella, M. G. Costa and A. R. Beltramone, Catal. Lett., 2001, 71, 127–131 CrossRef CAS.
- E. Gubbels, L. Jasinska-Walc and C. E. Koning, J. Polym. Sci., A: Polym. Chem., 2013, 51, 890–898 CrossRef CAS.
- J. Paciorek-Sadowska and B. Czupryński, J. Appl. Polym. Sci., 2006, 102, 5918–5926 CrossRef CAS.
- B. Toeroek, I. Bucsi, T. Beregszaszi, I. Kapocsi and A. Molnar, J. Mol. Catal. A: Chem., 1996, 107, 305–311 CrossRef CAS.
- A. Multer, N. McGraw, K. Hohn and P. Vadlani, Ind. Eng. Chem. Res., 2013, 52, 56–60 CAS.
- R. R. Emerson, M. C. Flickinger and G. T. Tsao, Ind. Eng. Chem. Prod. Res. Dev., 1982, 21, 473–477 CrossRef CAS.
- A. V. Tran and R. P. Chambers, Biotechnol. Bioeng., 1987, 29, 343–351 CrossRef CAS PubMed.
- (a) N. Xue, N. Liu, L. Nie, Y. Yu, M. Gu, L. Peng, X. Guo and W. Ding, J. Mol. Catal. A: Chem., 2010, 327, 12–19 CrossRef CAS; (b) N. Xue, X. Chen, L. Nie, X. Guo, W. Ding, Y. Chen, M. Gu and Z. Xie, J. Catal., 2007, 248, 20–28 CrossRef CAS.
- T. Blasco, A. Corma and J. Martínez-Triguero, J. Catal., 2006, 237, 267–277 CrossRef CAS.
- J. C. Vedrine, A. Auroux, P. Dejaifve, V. Ducarme, H. Hoser and S. Zhou, J. Catal., 1982, 73, 147–160 CrossRef CAS.
- (a) E. R. Alexander and D. C. Dittmer, J. Am. Chem. Soc., 1951, 73, 1665–1668 CrossRef CAS; (b) S. Itoh and H. Yamataka, J. Phys. Org. Chem., 2010, 23, 789–795 CAS.
- K. Mislow and M. Siegel, J. Am. Chem. Soc., 1952, 74, 1060–1061 CrossRef CAS.
- (a) C. P. Bezouhanova and F. A. Jabur, J. Mol. Catal., 1994, 87, 39–46 CrossRef CAS; (b) B. Y. Hsu and S. Cheng, Microporous Mesoporous Mater., 1998, 21, 505–515 CrossRef CAS.
- B. Sun, G. Yu, J. Lin, K. Xu, Y. Pei, S. Yan, M. Qiao, K. Fan, X. Zhang and B. Zong, Catal. Sci. Technol., 2012, 2, 1625–1629 CAS.
- T. Armaroli, L. J. Simon, M. Digne, T. Montanari, M. Bevilacqua, V. Valtchev, J. Patarin and G. Busca, Appl. Catal., A, 2006, 306, 78–84 CrossRef CAS.
- (a) M. A. Cambolor, A. Corma and J. Perez-Pariente, J. Chem. Soc., Chem. Commun., 1993, 557–559 RSC; (b) M. Ocaiia, V. FornCs and C. J. Serna, J. Non-Cryst. Solids, 1989, 107, 187 CrossRef.
- N. Y. Topsøe, K. Pedersen and E. G. Derouane, J. Catal., 1981, 70, 41–52 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |