Significant improvement in the hydrogen storage capacity of a reduced graphene oxide/TiO2 nanocomposite by chemical bonding of Ti–O–C†
Abstract
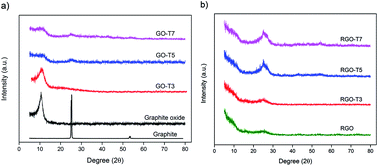
A series of graphene-based nanocomposites with different TiO2 contents have been prepared via a facile chemical method. All nanocomposites were employed as hydrogen gas adsorption materials at room temperature and pressures up to 10 bar. The effect of dispersion state and size of the particles on the hydrogen storage capacity of nanocomposites was studied. The highest hydrogen uptake of 0.39 wt% was obtained among prepared nanocomposites and it is 125% higher than the hydrogen adsorption of the parent graphene material. This improvement can account for the presence of a high number of active sites needed for hydrogen molecules and the strong interaction between nanoparticles and graphene sheets.

 Please wait while we load your content...
Please wait while we load your content...