Synthesis, characterization and photophysical properties of some 3,3′-bisindolyl(aryl)methanes†
Abstract
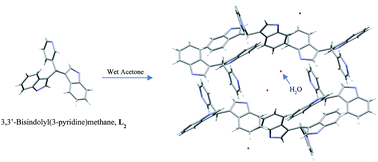
In this study, the successful synthesis of a series of 3,3′-bisindolyl(aryl)methanes, by modification of a known synthetic method in the literature, is reported. The main research goals included a detailed structural characterization of the products by means of NMR spectroscopy and X-ray crystallography, and understanding the role of aryl substituents on the photophysical properties of these compounds. Due to high solubility in the majority of polar solvents and insolubility in non-polar solvents, obtaining high quality crystals of these compounds was unsuccessful, leaving gaining data regarding bonds and angles in these compounds unattainable by X-ray crystallography. However, in one case, it was noticed that a polar solvent, such as water, can bind to an indole N–H group by establishing a hydrogen bond and a second indole N–H group forms another hydrogen bond to the neighboring pyridine nitrogen, creating one dimensional chains of 3,3′-bisindolyl(aryl)methanes. This type of bonding motif and the resulting conformational features illustrate how anion binding in these molecules is achieved. Such observations could be helpful in the study of selective ion sensing by these molecules. In the solution and solid-state absorption spectra of these compounds, two sets of high energy (220–290 nm) and low energy (490–530 nm) peaks are observed. The first set of peaks are attributable to allowed 1ππ* intraligand (1IL) transitions located on both the indole and R groups, and the second set may have resulted from intraligand and/or ligand to ligand charge transfer transitions (1LLCT). These highly emissive compounds display structured emission bands with a maximum at ca. 420–450 nm, originating from ligand centered 1ππ* emissions and contribution from both indole and R groups. In order to understand the nature of the absorptions, computations of the electronic absorption spectra using time-dependent DFT (TD-DFT) were carried out using the B3LYP method and a 6-311G* basis set for all atoms with the Gaussian 09 program. The solvent effect of dichloromethane in the TD-DFT calculations was also taken into account.

 Please wait while we load your content...
Please wait while we load your content...