Surface treatment and its effect on the electrochemical behavior of Ti–15Mo–3Nb–3Al alloy
R. V. Lakshmi,
Parthasarathi Bera* and
Chinnasamy Anandan
CSIR-National Aerospace Laboratories, Bangalore 560017, India. E-mail: partho@nal.res.in; Fax: +91-80-25210113; Tel: +91-80-25086359
First published on 30th March 2016
Abstract
The Ti–15Mo–3Nb–3Al alloy was subjected to four different surface treatments involving alkaline and acidic solutions. HNO3, HF–HNO3, NaOH etching and cathodic cleaning using NaOH were performed. The effect of these etchants on the surface morphology and the surface chemical composition was studied. Energy dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS) studies revealed the loss of oxygen content on the surface which indicated the partial breakdown of the surface oxide layer. Potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) curves showed the poor protection of the treated alloys against corrosion in 3.5% NaCl. Furthermore, these surfaces were coated with a silica–titania hybrid sol–gel film and the corrosion protection of the coated surfaces was assessed and compared. The results showed that sol–gel coating restored the corrosion resistance of all the treated surfaces.
1. Introduction
Titanium and its alloys are used in a wide range of applications from the biomedical field to aerospace industries because of their unique properties such as high tensile strength and toughness, light weight, good corrosion resistance, excellent biocompatibility and ability to withstand high temperatures. The aerospace industry is the largest market for titanium products primarily due to these exceptional properties. They are mostly utilized in jet engines, airframe components and other critical structure parts.1 Their basic advantages like high reliability during performance and good corrosion resistance make them a top choice for use in engines and airframes. However, a thorough surface modification process is needed prior to their usage. Surface modification process produces required specific surface topographies, cleans and roughens the surface in addition to enhancing several surface properties like adhesion in bonding, corrosion resistance, wear resistance etc.2 The prominent surface modification methods are mechanical, chemical, sol–gel, anodic oxidation, nitriding and ion implantation techniques.2,3 In recent years, different surface treatments of various Ti alloys and their effects on surface morphologies and corrosion behaviors in different solutions have been studied.2–16 Gostin et al. have shown that etching treatments of Ti–45Nb alloy in strongly oxidizing acidic solution like HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 (4
HNO3 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is found to be very effective for generating a nanopatterned surface topography which is expected to be promising for the stimulation of bone tissue growth.8 Acid pickling is a popular surface treatment method followed to clean substrate surface by removing oxides and contamination. A mixture of sulfuric acid and hydrogen peroxide has been used to eliminate surface contaminants and reported to produce a consistent and reproducible titanium oxide surface layer.9 Takeuchi and coworkers have studied etching efficiencies of Ti surface using acids such as Na2S2O8, H2SO4 and HCl.10 They have found that HCl is the most effective etching agent among the three because of its capability to dissolve titanium salts easily without weakening Ti surfaces. Alkali treatment by alkali solutions such as NaOH or KOH forms a bioactive porous layer on the substrate materials. Immersion of titanium alloy in a 5–10 M NaOH or KOH solution for 24 h is reported to form a porous layer of sodium titanate on the surface which has aided the formation of bone-like apatite layer.16
1) is found to be very effective for generating a nanopatterned surface topography which is expected to be promising for the stimulation of bone tissue growth.8 Acid pickling is a popular surface treatment method followed to clean substrate surface by removing oxides and contamination. A mixture of sulfuric acid and hydrogen peroxide has been used to eliminate surface contaminants and reported to produce a consistent and reproducible titanium oxide surface layer.9 Takeuchi and coworkers have studied etching efficiencies of Ti surface using acids such as Na2S2O8, H2SO4 and HCl.10 They have found that HCl is the most effective etching agent among the three because of its capability to dissolve titanium salts easily without weakening Ti surfaces. Alkali treatment by alkali solutions such as NaOH or KOH forms a bioactive porous layer on the substrate materials. Immersion of titanium alloy in a 5–10 M NaOH or KOH solution for 24 h is reported to form a porous layer of sodium titanate on the surface which has aided the formation of bone-like apatite layer.16
Sol–gel process is one of the popular surface modification methods carried out by depositing thin coatings on various substrates including titanium and its alloys. This technique is capable of producing coatings with controlled composition, microstructure and better chemical homogeneity. Low densification temperature and cost effectiveness are added advantages of this process. This method of surface modification has also been adopted by few researchers on titanium alloys.17–20 In the work of Liu et al., it has been demonstrated that glycidoxypropyl-trimethoxy-silane (GTMS) molecules are preferred to be adsorbed on β phase particles of Ti–6.5Al–1Mo–1V–2Zr alloy than α phase, especially at lower concentrations of GTMS which is due to the different chemical compositions and variation of the resulting surface hydroxylation in the studied solution.20 However, there is no study reported on the effect of surface modification from the corrosion aspect for titanium β–21S (Ti–15Mo–3Nb–3Al) alloy. Further, little is known about the passivity and corrosion resistance of this alloy in marine environment.21 In the present work, the effect of different alkali and acid treatments on the morphology and surface composition of uncoated alloy is presented by surface sensitive probes like 3D-profilometry, field emission scanning electron microscopy (FESEM) and X-ray photoelectron spectroscopy (XPS). The electrochemical behavior of the surface is investigated by potentiodynamic polarization and impedance measurements in 0.6 M NaCl medium. Coated alloy has been characterized by Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. Further, the effect of sol–gel coating in obviating the effects of surface treatments on corrosion resistance is presented.
2. Experimental details
2.1. Materials
Titanium β–21S (Ti–15Mo–3Nb–3Al–0.2Si) alloy was cut into small pieces of 1.5 × 1.5 × 0.2 cm3 size. The samples were smoothened by grinding with silicon carbide emery papers of different grit sizes (800, 1000 and 1200) and then polished to mirror-like finish using 0.03 μm alumina powder mixed with distilled water. The polished samples were ultrasonically cleaned in acetone for 15 min before any further use. Sol–gel precursors namely 3-glycidoxypropyltrimethoxysilane (GPTMS) and titanium isopropoxide (TIP) were supplied by Sigma-Aldrich and were used as-received. Acetylacetone, used as a complexing agent for TIP was also from Sigma-Aldrich. Ethanol (anhydrous, 99.5%) was used as the solvent and was from Changshu Yangyuan Chemicals, China. Nitric acid (HNO3, ∼70%) and sodium hydroxide (NaOH) were procured from Merck. Hydrofluoric acid (48%) was received from Sigma-Aldrich. Milli Q ultra pure water was used for the preparation of solutions.2.2. Surface treatment
The polished samples were chemically treated with different acidic and alkaline etchants prior to sol–gel coating deposition. Solutions of 20% HNO3, 0.5 M NaOH and HF–HNO3 mixture in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 volume with 40 mL water were prepared in Milli Q water and used as etchants. Acid pickled surfaces were obtained by immersing the substrates in HNO3 for 10 min. Similarly, alkaline treated surfaces were prepared by NaOH solution immersion for 10 min. Etching in HF–HNO3 mixture was limited to only 2 min. Cathodic cleaning was conducted by immersing the sample in 10% NaOH solution under an external bias of 2 V for 60 s. The sample acted as the cathode and a stainless steel strip was used as the anode. Then it was immersed in 10% H2SO4 for 30 s at ambient conditions. The treated surfaces were thoroughly washed with Milli Q water. They were then room temperature dried for about 15 min and then subjected to coating deposition.
10 volume with 40 mL water were prepared in Milli Q water and used as etchants. Acid pickled surfaces were obtained by immersing the substrates in HNO3 for 10 min. Similarly, alkaline treated surfaces were prepared by NaOH solution immersion for 10 min. Etching in HF–HNO3 mixture was limited to only 2 min. Cathodic cleaning was conducted by immersing the sample in 10% NaOH solution under an external bias of 2 V for 60 s. The sample acted as the cathode and a stainless steel strip was used as the anode. Then it was immersed in 10% H2SO4 for 30 s at ambient conditions. The treated surfaces were thoroughly washed with Milli Q water. They were then room temperature dried for about 15 min and then subjected to coating deposition.
2.3. Sol synthesis and deposition of coatings
GPTMS–TIP hybrid sol was synthesized by mixing two separately prepared precursor sols. GPTMS was magnetically stirred with ethanol in the presence of 0.36 mL 0.2 M HNO3 for 1 h. The molar ratio of silane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water was 1
water was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. TIP sol was prepared as the inorganic part by mixing TIP with acetylacetone, ethanol and water. TIP was first stirred with acetylacetone for 15 min in the molar ratio of 1
2. TIP sol was prepared as the inorganic part by mixing TIP with acetylacetone, ethanol and water. TIP was first stirred with acetylacetone for 15 min in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 prior to the addition of solvent. HNO3 was again added as the catalyst to promote hydrolysis. The molar ratio of GPTMS to TIP was 2
1 prior to the addition of solvent. HNO3 was again added as the catalyst to promote hydrolysis. The molar ratio of GPTMS to TIP was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. GPTMS sol was a clear colorless liquid while the TIP sol was a clear pale yellow liquid. Finally, after completing the hydrolysis, the two sols were mixed together and diluted with equal volume of ethanol. The final mixture was further stirred for 2 h for complete homogenization.
1. GPTMS sol was a clear colorless liquid while the TIP sol was a clear pale yellow liquid. Finally, after completing the hydrolysis, the two sols were mixed together and diluted with equal volume of ethanol. The final mixture was further stirred for 2 h for complete homogenization.
The prepared GPTMS–TIP hybrid sol was coated on to the treated substrates by dip-coating method (single dip coater, model SDC-2007C from Apex Instruments Co. Pvt. Ltd.). The process was carried out at a withdrawal speed of 90 mm min−1 after a residence time of 60 s in the sol. After coating, the samples were first dried at room temperature for 24 h and were subsequently heat-treated at 100 °C for 2 h in an air oven.
2.4. Surface characterization
The surface morphologies of the samples were examined by Carl Zeiss Supra 40 VP FESEM. The 3D surface images and 2D line profiles were obtained by NanoMap 500 LS 3D surface profilometer. Compositions of the samples were obtained by energy dispersive spectroscopy (EDS) using Oxford instruments Inca Penta FET X3 (Oxford Instruments, United Kingdom) attached to the FESEM.Structural characterization of the coating was studied by FTIR and Raman spectroscopy. FTIR spectrum was recorded using Bruker Alpha-P spectrometer. Raman data were obtained using Labram 010 Model of DILOR-JOBIN-YVON-SPEX Micro Raman spectrometer with 632 nm laser.
XPS of bare, chemically etched and sol–gel coated Ti alloys were recorded with a SPECS spectrometer, Germany using non-monochromatic AlKα radiation (1486.6 eV) as X-ray source operated at 150 W (12 kV, 12.5 mA). The binding energies reported here were referenced with C 1s peak at 284.6 eV with a precision of ±0.1 eV. All the survey spectra were obtained with a pass energy of 70 eV with step increment of 0.5 eV and individual spectra were recorded with a pass energy and step increment of 40 and 0.05 eV, respectively. For XPS analysis, samples were mounted on the sample holders and placed into a load-lock chamber with an ultrahigh vacuum (UHV) of 8 × 10−8 mbar for 5 h in order to desorb any volatile species present on the surface. After 5 h, samples were transferred into the analyzing chamber with UHV of 5 × 10−10 mbar. Ti 2p, Mo 3d and O 1s core level spectra were curve-fitted into their possible components using Gaussian–Lorentzian peaks after subtracting a Shirley background with CasaXPS program.
2.5. Electrochemical measurements
Corrosion studies on the substrate and coated samples were carried out using CH 604D electrochemical workstation supplied by CH instruments, USA. A conventional three electrode system was used to carry out the potentiodynamic polarization and EIS studies. The test sample was kept as the working electrode; Pt foil and saturated calomel electrode (SCE) connected to a Luggin capillary were used as counter and reference electrodes, respectively. NaCl solution of 3.5% was used as a corrosive medium to test the samples. The reference electrode was kept very close to the surface of the working electrode. The sample was immersed in NaCl solution till the open circuit potential (EOCP) is established (∼1 h). EIS measurement was carried out in the frequency range of 10 mHz to 100 kHz by applying a 10 mV sinusoidal wave. The impedance data were displayed as Nyquist and Bode plots. The acquired data were curve-fitted and analyzed using ZSimpwin program (Princeton Applied Research, USA) to get suitable equivalent circuit parameters. The quality of the fit was verified by the χ2 value. After EIS measurements, the system was allowed to attain OCP and subsequently polarization studies were carried out in the potential range of EOCP ±200 mV with a sweep rate of 1 mV s−1. The obtained potentiodynamic polarization plot (Tafel plot) was represented as potential vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i. The corrosion potential (Ecorr) and corrosion current (icorr) were deduced from the Tafel plot.
i. The corrosion potential (Ecorr) and corrosion current (icorr) were deduced from the Tafel plot.
3. Results and discussion
3.1. Microstructural studies
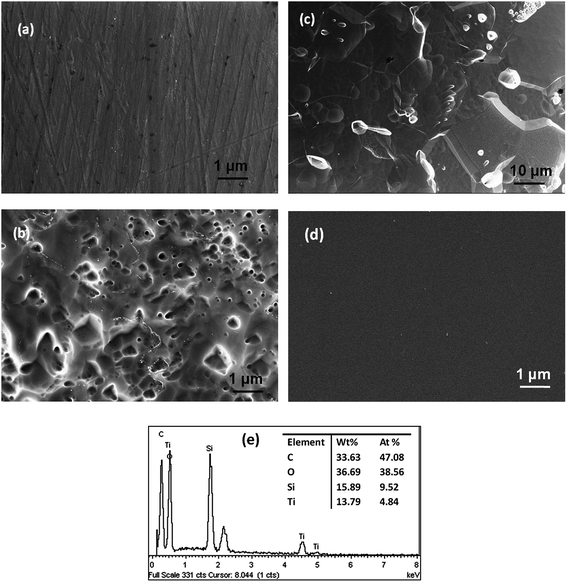
The surface microstructural features of the uncoated and coated samples were studied using 3D profilometer and FESEM. The 3D profiles of the uncoated surfaces after different chemical treatments are shown in Fig. 1. Samples in the as-polished condition with only acetone cleaning are used for comparison. The average roughness of the as-polished samples is 75 ± 10 nm. NaOH treated and cathodically cleaned surfaces show a roughness of about 86 nm and 66 nm, respectively, while a roughness of about 70 nm is observed in HNO3 treated surface. In the case of HF–HNO3 treated surface, the surface roughness increases to 208 nm. It is very evident from the profiles that surface treatment with NaOH and HNO3 including cathodic cleaning do not affect the surface roughness much. However, the highest roughness is seen for the HF–HNO3 treated surface which is increased almost by 2.7 times. The 3D image also shows that the surface morphology is severely changed and the grain structure is clearly seen. Thus, HF–HNO3 etching has substantially removed the top layers of the alloy including the protective oxide film.FESEM images of the acetone degreased and HF–HNO3 etched surfaces are shown in Fig. 2. As can be seen from the figures, acetone degreased sample shows few striations on the surface which must have resulted during surface grinding and polishing (Fig. 2a). The FESEM image of the HF–HNO3 etched surface at lower magnification (Fig. 2c) shows the grain structure. At higher magnification (Fig. 2b) lots of craters on the surface are seen which might be due to severe dissolution of the alloy in the strong etchant (Fig. 2b and c). Micrographs of the NaOH, HNO3 and cathodically cleaned surfaces (not shown here) appear very similar to acetone cleaned sample in their surface features. Thus, FESEM studies also support the 3D profilometer results that HF–HNO3 treatment leads to severe damage of the substrate surface. Fig. 2d shows a representative image of the sol–gel coating on the substrate in the as-prepared condition. The coating of about 4 μm thick covers all the surface features of the chemically treated alloy samples. Since all the samples exhibit smooth and uniform surface, only one image is shown for representation.
3.2. EDS studies
Compositional analysis of the substrate in the as-received condition as well as after chemical treatment has been carried out by EDS. Compositional analysis of the coating has also been done. It is to be noted that thickness of the Ti alloy substrate is 1.5 mm and that of the coating is 4 μm. Energy of 10 keV is used for EDS experiment and penetration depth of electrons with this energy is less than 1 μm. Therefore, sampling depth for EDS experiment is much less than sample and coating thickness. The substrate shows Al, Si, Nb and Mo as the alloying elements with their wt% of 2.47, 0.58, 3.19 and 15.74, respectively and the balance being Ti. The alloy also shows an oxygen concentration of 4.7 wt%. The compositions of the treated substrates are as shown in Table 1. The concentrations of Al and Nb are varied marginally on different treatments. There is a slight enrichment in the Mo content. The concentration of oxygen on the samples decreases on the subjection to chemical treatments. It is about 2.5 wt% for the HNO3 and cathodically cleaned surface and only about 1 wt% for NaOH treated surface. HF–HNO3 shows no traces of oxygen on the surface. ED spectrum for the sol–gel coated sample is shown in Fig. 2e. The concentration of Si and Ti are 9.5 and 4.8 at% which reaffirms the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of GPTMS and TIP.
1 molar ratio of GPTMS and TIP.
| Alloys | Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Si | Nb | Mo | O | V | Ti | |
| Acetone degreased | 2.47 | 0.58 | 3.19 | 15.74 | 4.73 | — | 73.29 |
| NaOH treated | 2.65 | — | 2.66 | 17.19 | 0.98 | 0.46 | 76.06 |
| HNO3 treated | 2.71 | — | 3.69 | 17.67 | 2.43 | 0.36 | 73.15 |
| HF–HNO3 treated | 2.70 | — | 3.94 | 16.99 | — | — | 76.37 |
| Cathodically cleaned | 2.57 | 0.81 | 2.84 | 16.16 | 2.58 | — | 75.04 |
3.3. FTIR and Raman studies
The chemical characterization of coating has further been performed by vibrational spectroscopy techniques, namely FTIR and Raman. The corresponding spectra of coated alloy are shown in Fig. 3a and b, respectively. In the FTIR spectrum (Fig. 3a), a broad peak at 3470 cm−1 and small peak at 1629 cm−1 correspond to O–H bonds. The vibrational band around 2850 cm−1 is attributed to the C–H vibrations in the glycidoxypropyl group of GPTMS and acetylacetone.22,23 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of acetylacetone can occur at 1630 cm−1. Characteristic band for epoxide ring vibrations of GPTMS is seen at 1257 cm−1. Strong bands observed at 1100–1130 cm−1 are characteristic of Si–O–Si moieties.24 At lower wavenumbers, asymmetric stretching vibration of Si–O–Si is seen at around 900 cm−1. The data, thus suggest the formation of a hybrid network between GPTMS and TIP.
O stretching vibrations of acetylacetone can occur at 1630 cm−1. Characteristic band for epoxide ring vibrations of GPTMS is seen at 1257 cm−1. Strong bands observed at 1100–1130 cm−1 are characteristic of Si–O–Si moieties.24 At lower wavenumbers, asymmetric stretching vibration of Si–O–Si is seen at around 900 cm−1. The data, thus suggest the formation of a hybrid network between GPTMS and TIP.
In the Raman spectrum (Fig. 3b), the strong peak at 456 cm−1 originates from –(Si–O–Si) bending. The weak signal seen in the spectrum at 644 cm−1 is due to symmetric stretching of –(Si(–O–CH3)3) moieties. This shift is characteristic of partially unhydrolyzed trimethoxysilane molecules of GPTMS. The characteristic Raman shift of GPTMS is located at 1264 cm−1 and is assigned to epoxy ring breathing. The peak at 1293 cm−1 is due to (–(CH2)n–) wagging. The intensity at 1455 cm−1 is assigned for asymmetric bending modes of δ(CH3), δ(CH2) or δ(CH) groups. The vibrations at 1033, 1058 and 1409 cm−1 are related to the C–C skeletal vibration or ν(Si–O) stretching in inorganic network.25 The vibration at 848 cm−1 corresponds to oxirane symmetric deformation.26 The asymmetric stretching of Si–O–Si linkage occurs at 1196 cm−1. A Raman shift at 980 cm−1 is of the Si–O stretching vibration of silanol (Si–OH) groups.
3.4. XPS studies
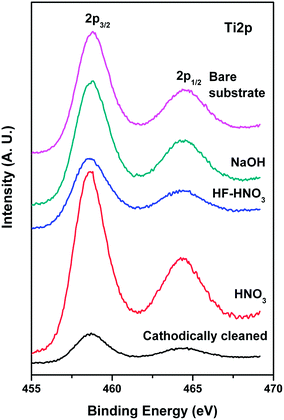
Detailed XPS characterization has been carried out to understand the surface nature of Ti alloys with different conditions. Typical XPS survey spectra of Ti alloys with different etching conditions are given in Fig. 4. They clearly show the presence of Ti, Mo and O species in all the alloys. Ti, O and Si are observed to be present in sol–gel coated Ti alloy. In Table 2, surface concentrations of Ti, Mo and Nb are given. It has been observed that Mo amount increases in the alloys with etching treatment in HF–HNO3 and in cathodically cleaned alloy in comparison with bare substrate alloy. However, there is not much change in Mo amounts in NaOH and HNO3 treated alloys. Ti 2p core level spectra of bare Ti substrate and etched alloys have been recorded and are displayed in Fig. 5. Bare Ti substrate shows Ti 2p3/2,1/2 peaks at 458.6 and 464.2 eV that correspond to Ti4+ species.27 Ti is observed to be present in +4 oxidation state in the NaOH, HF–HNO3 and HF etched and cathodically cleaned alloys. There is a small component peak around 457.5 eV that related to Ti3+ species in all alloys. Mo 3d core level spectra of the treated alloys are shown in Fig. 6. Spectral envelops of Mo 3d core levels are broad in nature indicating that Mo is present in different oxidation states and it can be curve-fitted into sets of spin–orbit doublets. Typical curve-fitted Mo 3d core level spectrum of the alloy etched with HF–HNO3 is given in Fig. 7. Observed Mo 3d5/2 peaks at 227.6, 229.5, 231.1 and 232.5 eV in all alloys are assigned to Mo0, Mo4+, Mo5+ and Mo6+ species, respectively.28,29 However, amount of metal is found to be less in cathodically cleaned and HNO3 treated alloys compared to bare substrate alloy, whereas metal concentration is more in HF–HNO3 etched alloys. Nb is present in +5 oxidation state in all alloys. Binding energies, surface concentrations of component species of Ti and Mo in the treated alloys evaluated from XPS studies are summarized in Table 3. XPS of O 1s core levels of alloys treated with different conditions are broad in nature that can be curve-fitted into several component peaks. Main peak around 530.2 eV is attributed to oxide species present in the alloys and the observed peaks around 531.8 and 533.5 eV correspond to oxygen associated with hydroxyl (OH−) species and H2O.30 A typical curve-fitted O 1s core level spectrum of the alloy etched with HNO3 is shown in Fig. 8.| Alloys | Ti | Mo | Nb |
|---|---|---|---|
| Acetone degreased | 87.1 | 10.9 | 2.0 |
| NaOH treated | 88.4 | 9.4 | 2.2 |
| HNO3 treated | 87.6 | 10.3 | 2.1 |
| HF–HNO3 treated | 74.9 | 22.3 | 2.8 |
| Cathodically cleaned | 84.5 | 13.3 | 2.2 |
 | ||
| Fig. 7 Curve-fitted Mo 3d core level spectrum of Ti–15Mo–3Nb–3Al alloy surface treated with HF–HNO3. | ||
| Alloys | Ti species | Binding energy of Ti 2p3/2 (eV) | Relative peak area (%) | Mo species | Binding energy of Mo 3d5/2 (eV) | Relative peak area (%) |
|---|---|---|---|---|---|---|
| Bare alloy | Ti3+ | 457.6 | 8 | Mo0 | 227.4 | 11 |
| Ti4+ | 458.8 | 92 | Mo4+ | 229.3 | 33 | |
| Mo5+ | 231.1 | 28 | ||||
| Mo6+ | 232.4 | 28 | ||||
| NaOH treated | Ti3+ | 457.5 | 12 | Mo0 | 227.7 | 13 |
| Ti4+ | 458.5 | 88 | Mo4+ | 229.4 | 46 | |
| Mo5+ | 231.5 | 19 | ||||
| Mo6+ | 232.7 | 22 | ||||
| HNO3 treated | Ti3+ | 457.7 | 10 | Mo0 | 227.6 | 8 |
| Ti4+ | 458.5 | 90 | Mo4+ | 229.5 | 30 | |
| Mo5+ | 231.1 | 30 | ||||
| Mo6+ | 232.7 | 32 | ||||
| HF–HNO3 treated | Ti3+ | 457.6 | 13 | Mo0 | 227.6 | 15 |
| Ti4+ | 458.6 | 87 | Mo4+ | 229.5 | 28 | |
| Mo5+ | 231.1 | 30 | ||||
| Mo6+ | 232.7 | 27 | ||||
| Cathodically cleaned | Ti3+ | 457.7 | 8 | Mo0 | 227.6 | 8 |
| Ti4+ | 458.8 | 92 | Mo4+ | 229.2 | 15 | |
| Mo5+ | 231.0 | 25 | ||||
| Mo6+ | 232.5 | 52 |
3.5. Electrochemical studies
 | ||
| Fig. 9 Potentiodynamic polarization curves of (a) chemically treated uncoated substrates and (b) sol–gel coated Ti–15Mo–3Nb–3Al alloys in 3.5% NaCl solution. | ||
| Alloys | Uncoated samples | Coated samples | ||
|---|---|---|---|---|
| icorr (μA cm−2) | Ecorr (V) | icorr (μA cm−2) | Ecorr (V) | |
| Acetone degreased | 0.04 | −0.3 | 0.06 | −0.22 |
| NaOH treated | 1.2 | −0.33 | 0.08 | −0.25 |
| HNO3 treated | 0.25 | −0.43 | 0.08 | −0.19 |
| HF–HNO3 treated | 2 | −0.41 | 2.5 | −0.29 |
| Cathodically cleaned | 1.6 | −0.35 | 0.02 | −0.25 |
Sol–gel coating on acetone degreased surface shows icorr value of 0.06 μA cm−2 (Fig. 9b) which is almost same as that in uncoated condition. However, it is worth to note from the figure that the anodic curve for the coated sample is smoother and horizontal compared to the uncoated substrate. This is a clear indication that the passive behavior of the surface is indeed enhanced by the sol–gel coating thereby acting like an additional barrier layer. Surfaces with different chemical treatments also exhibit very low icorr values (0.02–0.08 μA cm−2) on sol–gel coating. Thus, the coating provides a barrier protection to the surface against the ingress of electrolyte and prevents the attack by the chloride ions. The Ecorr values for the coated samples fall in a very narrow range of −0.19 to −0.25 V and this further confirms the protection rendered by the coating. But, HF–HNO3 treated surface does not show any major improvement in spite of sol–gel coating. The defects caused by surface etching can be a reason for this which is understood from their microstructural features and surface composition as discussed previously.
 | ||
| Fig. 10 Electrochemical impedance curves: (a) Bode plot and (b) phase angle plot of chemically treated uncoated Ti–15Mo–3Nb–3Al alloys in 3.5% NaCl solution. | ||
 | ||
| Fig. 11 Electrochemical impedance curves: (a) Bode plot and (b) phase angle plot of sol–gel coated Ti–15Mo–3Nb–3Al alloys in 3.5% NaCl solution. | ||
The corrosion protection efficiency of a system is usually assessed from the impedance values obtained at the low frequency region; higher the value better is the protection. From Fig. 10, acetone degreased substrate shows the highest value of impedance compared to all other chemically treated surfaces. This increased protection is definitely from the naturally formed oxide layer which is intact. In all other cases, the drop in the impedance value indicates the partial breakdown of the protective layer and initiation of corrosion activity at the metal surface. A broad phase angle peak seen for the acetone degreased surface is indicative of the capacitive behavior of protective oxide layer and its distributed pore sizes. Reduction in the peak width, observed for the chemically treated surfaces at ∼11 Hz can imply thinning of the oxide layer. Decrease in the peak height is an indication of deterioration in capacitive behavior of the oxide layer. A second low frequency time constant which is an indication of corrosion attack is well defined for the HF–HNO3 treated surface. This is because, HF being a strong etchant for the alloy, has destroyed the oxide layer almost completely and thus the surface is immediately attacked in the NaCl solution. Whereas in the other cases, it is observed that although the drop in the impedance modulus is to the same extent, the time constant corresponding to the corrosion activity is not so well defined. Thus, the plots confirm that all the used chemical treatments partially destroy the native oxide film on the metal surface and hence affects it inherent corrosion resistant property.
For the sol–gel coated samples, the variation in the impedance values at low frequency region is not very significant (Fig. 11). This suggests that the sol–gel layer offers uniform improved protection to the surface. In fact, sol–gel coating on HNO3 treated surface gives the maximum protection to the surface with an impedance modulus of about 1 × 106 Ω cm2. However, sol–gel coating on HF–HNO3 treated surface shows the lowest protection with a value of almost one order less. There is not much difference between the performances of other three surfaces except some minimal changes. The EIS curves obtained for the sol–gel uncoated and coated samples have been suitably fitted using equivalent circuit shown in Fig. 12 and the results obtained are summarized in Tables 5 and 6, respectively. The fitted curves are also shown in the respective impedance plots. Good conformity has been obtained between the fitted and experimental data in all the cases and quality of the fit has been checked by the χ2 value. The non-ideal capacitive response of the oxide films has been entailed by using a constant phase element (CPE) instead of a pure capacitance in the fitting procedure. This CPE can be due to difference in the relaxation times as a result of different degrees of surface in-homogeneity, roughness factors and compositions of surface layers. The impedance with the capacitances can be defined as ZCPE = 1/Q(jω)n, where Q, j, ω and n are the pseudo capacitance, imaginary function (√−1), angular frequency and the deviation from the ideal behavior of a pure capacitor, respectively. When n = 1, the system behaves like a pure capacitor and Q = C where C is capacitance.
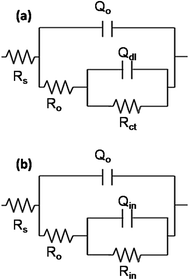 | ||
| Fig. 12 Electrochemical equivalent circuits used to fit the EIS data of Ti–15Mo–3Nb–3Al alloys with different conditions. | ||
| Alloys | Qcoat (S sn cm−1) | ncoat | Rcoat (Ω cm2) | Qdl (S sn cm−1) | ndl | Rct (Ω cm2) |
|---|---|---|---|---|---|---|
| Acetone degreased | 2.2 × 10−5 | 0.94 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.54 × 10−5 | 0.88 | 2.7 × 106 |
| NaOH treated | 2.9 × 10−5 | 0.92 | 6922 | 22 × 10−5 | 0.57 | 2.5 × 104 |
| HNO3 treated | 2.6 × 10−5 | 0.94 | 7189 | 17 × 10−5 | 0.83 | 6.6 × 105 |
| HF–HNO3 treated | 4.1 × 10−5 | 0.89 | 5593 | 28 × 10−5 | 0.8 | 3.6 × 104 |
| Cathodically cleaned | 1.8 × 10−5 | 0.91 | 8300 | 0.67 × 10−5 | 0.73 | 8.7 × 104 |
| Alloys | Qcoat (S sn cm−1) | ncoat | Rcoat (Ω cm2) | Qox (S sn cm−1) | nox | Rox (Ω cm2) |
|---|---|---|---|---|---|---|
| Acetone degreased | 2.72 × 10−9 | 0.96 | 332.8 | 2.1 × 10−5 | 0.91 | 1.54 × 107 |
| NaOH treated | 2.30 × 10−8 | 0.83 | 169.0 | 4.07 × 10−5 | 0.92 | 5.1 × 106 |
| HNO3 treated | 3.40 × 10−9 | 0.98 | 111.2 | 1.75 × 10−5 | 0.91 | 1.65 × 107 |
| HF–HNO3 treated | 4.20 × 10−6 | 0.63 | 46.6 | 3.67 × 10−5 | 0.85 | 1.59 × 105 |
| Cathodically cleaned | 1.3 × 10−5 | 0.92 | 107.0 | 1.95 × 10−5 | 0.90 | 1.45 × 107 |
The equivalent circuit shown in Fig. 12a describes the surface of uncoated chemically treated samples and the obtained parameters are summarized in Table 5. Here, Rs is the solution resistance, RO and QO are the resistance and pseudo capacitance associated with the penetration of electrolyte through the porous oxide layer. Similarly, Rct and Qdl represent the resistance and pseudo capacitance of the charge transfer reactions happening at the substrate/electrolyte interface. The value of RO for the as-received acetone degreased sample is 1.2 × 104 Ω cm2. The alkaline and HNO3 treated samples have the porous oxide layer showing resistance values relatively low in the range 7–8 kΩ cm2. The HF–HNO3 treated sample shows the lowest resistance of 5593 Ω cm2, against the penetration of electrolyte. This suggests that HF–HNO3 etchant damages the outer porous protective layer (reduces the thickness) and permits the ingress of electrolyte. Rct is also seen to be highest for the acetone cleaned sample compared to the other samples.
The impedance data of the coated samples are fitted with the circuit shown in Fig. 12b. The obtained parameters are tabulated in Table 6. In the circuit, RO and QO correspond to the resistance and pseudo capacitance of the porous sol–gel layer and Rin and Qin to that of the inner dense oxide layer. The dense oxide layer is formed from the interaction between Ti–OH groups of the substrate and Si–OH, Ti–OH groups of the sol, thus forming Ti–O–Si and Ti–O–Ti covalent bonds. Comparing the values of RO, it is evident that the pore resistance of sol–gel coating on acetone degreased surface is about 2–3 times higher than those on the chemically treated samples. The low values of RO indicate the attack of the coating surface by chloride ions. The value of Rin is highest for HNO3 treated sample (2.5 × 107 Ω cm2). RO values of acetone degreased and cathodically cleaned sol–gel coated samples are in the same order (1.5 × 107 Ω cm2). The coated sample with HF–HNO3 treatment has the lowest RO value of 46 Ω cm2 and also exhibits the oxide resistance lower by about 2 orders (1.6 × 105 Ω cm2). Thus, the protection to the surface has been rendered by the dense layer of the oxide film and that from the porous top layer is negligible. The value of n2 being 0.9 (closer to 1) further confirms the intactness of the layer.
As seen from above results among all surface treated alloys, lowest corrosion current is observed for HNO3 treated samples. HNO3, being a good oxidizing agent may help passivation of the surface thereby being less detrimental to it. Also Mo is known to show better corrosion resistance behavior. XPS studies have demonstrated that oxidized Mo is observed to be more in HNO3 etched coating compared to other treated coatings (Table 3). On the other hand, NaOH and HF–HNO3 treated alloys contain less oxidized Mo according to XPS studies. 3D profilometry studies also show that HF–HNO3 treatment removes passive oxide layer on the top surface. Hence, corrosion resistance property of HF–HNO3 treated alloy is low compared to other alloys. Sol–gel coating removes the effects of all the surface treatments and restores the corrosion resistance of the treated surface except in the case of HF–HNO3 treated one. This may be due to poorer condition of the initial surface.
4. Conclusions
Titanium alloy (Ti–15Mo–3Nb–3Al) has been chemically treated with different reagents including NaOH, HNO3 and HF–HNO3 solutions. The morphological study indicates that the surface has severely been affected by the HF–HNO3 etchant. Other treatments do not bring about any major changes. EDS and XPS studies reveal the partial breakdown of surface oxide layer. HNO3 treated alloy surface gives a better corrosion protection to the surface because of its passivation nature and HF–HNO3 treated alloy shows the least protection. Sol–gel coatings on the other hand, provide improved protection to all the surfaces and this is because of the formation of a dense sol–gel oxide layer due to the interaction between Ti–OH groups of the substrate and Si–OH, Ti–OH groups of the sol, thereby forming Ti–O–Si and Ti–O–Ti covalent bonds.Acknowledgements
Authors wish to thank Director, CSIR-National Aerospace Laboratories for giving permission to publish this work. Help rendered by Mr Siju and Mr Praveen for FESEM and 3D profilometry studies is gratefully acknowledged.References
- M. Peters, J. Kumpfert, C. H. Ward and C. Leyens, Adv. Eng. Mater., 2003, 5, 419 CrossRef CAS.
- S. Izman, M. R. Abdul-Kadir, M. Anwar, E. M. Nazim, R. Rosliza, A. Shah and M. A. Hassan, Titanium Alloys – Towards Achieving Enhanced Properties for Diversified Applications, in Surface Modification Techniques for Biomedical Grade of Titanium Alloys: Oxidation, Carburization and Ion Implantation Processes, ed. Dr A. K. M. N. Amin, InTech, Rijeka, Croatia, 2012 Search PubMed.
- X. Liu, P. K. Chu and C. Ding, Mater. Sci. Eng., R, 2004, 47, 49 CrossRef.
- A. M. Kumar, P. Sudhagar, S. Ramakrishna, Y. S. Kang, H. Kim, Z. M. Gasem and N. Rajendran, Appl. Surf. Sci., 2014, 307, 52 CrossRef CAS.
- A. Kazek-Kęsik, M. Krok-Borkowicz, E. Pamuła and W. Simka, Mater. Sci. Eng., C, 2014, 43, 172 CrossRef PubMed.
- H.-H. Huang, C.-P. Wu, Y.-S. Sun, H.-M. Huang and T.-H. Lee, Thin Solid Films, 2013, 549, 87 CrossRef CAS.
- Y. Sasikumar, M. Karthega and N. Rajendran, J. Mater. Eng. Perform., 2011, 20, 1271 CrossRef CAS.
- P. F. Gostin, A. Helth, A. Voss, R. Sueptitz, M. Calin, J. Eckert and A. Gebert, J. Biomed. Mater. Res., Part B, 2013, 101, 269 CrossRef PubMed.
- A. Nanci, J. D. Wuest, L. Peru, P. Brunet, V. Sharma, S. Zalzal and M. D. McKee, J. Biomed. Mater. Res., 1998, 40, 324 CrossRef CAS PubMed.
- M. Takeuchi, Y. Abe, Y. Yoshida, Y. Nakayama, M. Okazaki and Y. Akagawa, Biomaterials, 2003, 24, 1821 CrossRef CAS PubMed.
- Y.-L. Zhou and D.-M. Luo, J. Alloys Compd., 2011, 509, 6267 CrossRef CAS.
- H. Krawiec, V. Vignal, J. Loch and P. Erazmus-Vignal, Corros. Sci., 2015, 96, 160 CrossRef CAS.
- R. Mythili, S. Saroja and M. Vijayalakshmi, Mater. Charact., 2010, 61, 1326 CrossRef CAS.
- J. Jayaraj, A. R. Shankar and U. K. Mudali, Electrochim. Acta, 2012, 85, 210 CrossRef CAS.
- Y. Yang, C. Xia, Z. Feng, X. Jiang, B. Pan, X. Zhang, M. Ma and R. Liua, Corros. Sci., 2015, 101, 56 CrossRef CAS.
- H.-M. Kim, F. Miyaji, T. Kokubo and T. Nakamura, J. Biomed. Mater. Res., Part A, 1996, 32, 409 CrossRef CAS.
- S. M. Bhola and B. Mishra, Int. J. Electrochem. Sci., 2013, 8, 7075 CAS.
- R. Roest, A. J. Atanacio, B. A. Latella, R. Wuhrer and B. Ben-Nissan, Mater. Forum, 2007, 31, 160 CAS.
- M. C. Advincula, D. Petersen, F. Rahemtulla, R. Advincula and J. E. Lemons, J. Biomed. Mater. Res., Part B, 2007, 80, 107 CrossRef PubMed.
- J.-H. Liu, Z.-W. Zhan, M. Yu, S.-M. Li, Q. Wang, J. Zhang and M. Wang, Surf. Interface Anal., 2012, 44, 863 CrossRef CAS.
- D. G. Kolman and J. R. Scully, J. Electrochem. Soc., 1993, 140, 2771 CrossRef CAS.
- R. V. Lakshmi, T. Bharathidasan and B. J. Basu, Appl. Surf. Sci., 2011, 257, 10421 CrossRef CAS.
- R. V. Lakshmi, G. Yoganandan, K. T. Kavya and B. J. Basu, Prog. Org. Coat., 2013, 76, 367 CrossRef CAS.
- A. Jitianu, J. Doyle, G. Amatucci and L. C. Klein, J. Sol-Gel Sci. Technol., 2010, 53, 272 CrossRef CAS.
- M. Gnyba, M. Keränen, M. Kozanecki, R. Bogdanowicz, B. B. Kosmowski and P. Wroczynski, Opto-Electron. Rev., 2002, 10, 137 CAS.
- B. Riegel, W. Kiefer, S. Hofacker and G. Schottner, J. Sol-Gel Sci. Technol., 2002, 24, 139 CrossRef CAS.
- M. Sreedhar, I. N. Reddy, P. Bera, D. Ramachandran, K. G. Saravanan, A. M. Rabel, C. Anandan and P. Kuppusami, Appl. Phys. A, 2015, 120, 765 CrossRef CAS.
- S. I. Castañeda, I. Montero, J. M. Ripalda, N. Díaz, L. Galán and F. Rueda, J. Appl. Phys., 1999, 85, 8415 CrossRef.
- M. Tordjman, C. Saguy, A. Bolker and R. Kalish, Adv. Mater. Interfaces, 2014, 1, 1300155 Search PubMed.
- P. Bera, H. Seenivasan and K. R. Rajam, Surf. Rev. Lett., 2013, 20, 1350006 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |