Isotype heterostructure of bulk and nanosheets of graphitic carbon nitride for efficient visible light photodegradation of methylene blue
Biswajit Choudhurya and
P. K. Giri*ab
aDepartment of Physics, Indian Institute of Technology Guwahati, Guwahati 781039, India. E-mail: giri@iitg.ernet.in
bCenter for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati 781039, India
First published on 1st March 2016
Abstract
Nanosheets of g-C3N4 were prepared by the ultrasonic treatment of aqueous dispersed bulk C3N4 for 10 h. The nanosheets have a comparatively larger surface area (121 m2 g−1) than that of bulk C3N4 (18 m2 g−1). Bulk C3N4 prepared by direct heating of urea has a band gap of 2.74 eV, whereas the nanosheets of g-C3N4 exhibited an enlarged band gap of 2.97 eV. The isotype heterostructure is fabricated by the solid-state mixing of bulk and nanosheets of C3N4 followed by ultrasonic treatment for dispersion. The heterostructure shows an effective band gap of 2.62 eV with an average charge carrier lifetime of 21 ns, which is longer than that of the bulk (13.2 ns) or nanosheets (17.4 ns) of g-C3N4. The heterostructure exhibits significantly higher visible light photocatalytic activity in the degradation of methylene blue (MB) over bulk or nanosheets of g-C3N4. The superior photocatalytic performance of the heterostructure is ascribed to band-bending at the interface that promotes molecular exciton dissociation and facilitates facile separation of charge carriers at the interface. From the results of photocatalysis, it is speculated that the photogenerated ˙OH radicals in conjunction with ˙H atoms take part in photocatalysis by N-deethylation followed by aromatic ring cleavage of the MB molecule.
1. Introduction
Heterogeneous photocatalysis based on TiO2 has been researched for a few decades, owing to its stable photochemical reactions in the detoxification of air/water pollutants and H2 generation by water splitting by utilizing the UV part of solar spectrum.1,2 Practical applications of TiO2 are substantially compromised by its wide band gap with limited or no visible absorption. Recently, a metal free polymeric semiconductor, graphitic carbon nitride (g-C3N4), has drawn immense interest because of its promising applications in H2 generation, photodegradation of organic pollutants, and possible optoelectronic applications.3–5 The basic unit of g-C3N4 is the tri-s-triazine unit (or s-heptazine) with a strong C–N covalent bond, and the layers in g-C3N4 are connected via van der Waals interactions.6,7 The electronegativity difference between C and N in the s-heptazine ring could possibly results in the opening up of a band gap in g-C3N4, and as reports predict the measured bulk band gap of g-C3N4 is ∼2.7 eV with valence and conduction band edge comprising of N 2p lone pair orbital and C 2p orbital, respectively.8,9 The preparation method of g-C3N4 is simple and involves pyrolysis of nitrogen rich precursors, viz., cyanamide, thiourea, urea at different processing temperatures.10 Although the thermally processed g-C3N4 shows visible light photocatalytic activity, it exhibits less surface area, only marginal absorption in visible region and suffer from high probability of bulk recombination of photoexcited charge carriers.11 Ultrathin nanosheets of g-C3N4 are fabricated by liquid phase exfoliation in solvents n-methyl pyrrolidone, 2-propanol, water, etc.12 The nanosheets have tunable absorption and prolonged carrier lifetime, but shows an enlargement in band gap with respect to bulk C3N4.13,14 As such, for practical applications, the optical absorption in g-C3N4 should be improved with narrowed band gap and prolonged lifetime of charge carrier separation. This is indeed achieved by introducing heteroatoms (B, S, O) and constructing heterostructures with other semiconductors (e.g. ZnO) with interface mediated charge carrier separation.4,15,16 In case of doping, control of doping is a necessary step; otherwise dopants itself may act as carrier recombination center. In contrast, in case of hybridization with inorganic semiconductors proper band alignment and interface formation is essential for efficient charge separation.Zhang et al.17 developed a metal free isotype heterostructure by the pyrolysis of mixtures of thiourea (S, N, C source) and urea (C, N source). The as-formed heterostructure exhibited very high photocatalytic activity because of different band alignment at the interface promoting charge separation. Dong et al.18 constructed type I and type II isotype heterostructure with different band alignment at the interface, and studied the influence of band bending and charge carrier separation on the effective photocatalytic removal of NO pollutant from air. The benefit of constructing heterostructure of g-C3N4 with graphene or other metal oxides has also been considered useful for several energy and environmental applications.19 In the present study, we have developed an isotype heterostructure starting with the same urea precursors. Initially, bulk g-C3N4 is prepared by thermal condensation of urea. The product is subjected to aqueous phase ultrasonication treatment to yield nanosheets of g-C3N4. Bulk and nanosheets of g-C3N4 are mixed by solid-state mixing and ultrasonically treated to obtain isotype-heterostructure of g-C3N4. Bulk, nanosheet and heterostructure samples are characterized with XRD, FTIR, UV-vis, photoluminescence (PL) spectroscopic techniques. Aqueous exfoliated C3N4 nanosheets exhibit very good photodegradation of MB, because of its high surface area and largely separated charge carriers than that of bulk C3N4. Interestingly, heterostructure shows impressively higher absorption in visible region due to effectively lower band gap, and prolonged separation of charge carriers with excellent photocatalytic activity as compared to bulk or nanosheets of g-C3N4.
2. Experimental details
2.1 Preparation of bulk, nanosheets and isotype heterostructure of g-C3N4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to obtain graphitic carbon nitride nanosheet. The sample was labeled as NCN (nanosheets of g-C3N4).
000 rpm to obtain graphitic carbon nitride nanosheet. The sample was labeled as NCN (nanosheets of g-C3N4).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of BCN and NCN was grounded in an agate mortar. Solid state mixing in agate mortar was continued for 1 h. The mixture of BCN and NCN was transferred to a beaker. Then 100 mL H2O was added to the beaker and stirred for 1 h. The stirred mixture was then transferred to an ultrasonicator and ultrasonicated for 1 h. After ultrasonication the mixture was naturally dried at 70 °C. This as-prepared sample was labeled as BCN–NCN.
1) of BCN and NCN was grounded in an agate mortar. Solid state mixing in agate mortar was continued for 1 h. The mixture of BCN and NCN was transferred to a beaker. Then 100 mL H2O was added to the beaker and stirred for 1 h. The stirred mixture was then transferred to an ultrasonicator and ultrasonicated for 1 h. After ultrasonication the mixture was naturally dried at 70 °C. This as-prepared sample was labeled as BCN–NCN.2.2 Characterization details
High resolution X-ray diffraction (XRD) pattern was obtained in a Bruker D8 focus AXS X-ray diffractometer equipped with a Cu Kα source of λ = 1.54 Å. Morphology of prepared nanosheet was monitored in ZEOL JEM 200 kV transmission electron microscope. Fourier transform infrared spectroscopy (FTIR) was performed in a Nicolet I-410 FTIR spectrophotometer using KBr pellet. N2 adsorption–desorption isotherm was obtained at 77 K in a Quantachrome iQ autosorb analyzer. For the determination of surface area and pore size distribution from the isotherm, we followed multipoint Brunauer–Emmett–Teller (BET) method and Barett–Joyner–Halenda (BJH) model, respectively. UV-vis diffuse reflectance (DRS) spectra were recorded in Shimadzu 2450 UV-vis spectrophotometer. Steady state photoluminescence (PL) spectra were recorded in Fluoromax-4 (Horiba Scientific) spectrophotometer. Time resolved photoluminescence (TRPL) analysis was carried out in picosecond time resolved luminescence spectrometer (Edinburg Instruments, Model: FSP920). TRPL data was obtained by exciting the sample at 375 nm.2.3 Photocatalytic activity study
Photodegradation of methylene blue (MB) by g-C3N4 was studied by monitoring the decrease in the initial concentration of MB solution on exposure to visible light (250 W) for different time intervals. Photocatalytic reaction was performed by dispersing 30 mg of g-C3N4 catalyst in 100 mL MB solution with an initial MB concentration of 8 mg L−1. Before visible light exposure, MB solution with catalyst was stirred in dark for 45 min. This allowed complete adsorption of dye molecule on the g-C3N4 surface and equilibrated the adsorption–desorption process on the catalyst surface. Absorption measurement of blank and catalyst loaded MB solution was measured in absence of light. The reactant solution was then placed at a distance of 5 cm from the visible light source (390–730 nm). Light exposure on the MB solution was continued for 90 min. After each 15 min interval, 10 mL of the MB solution was taken out and centrifuged. The suspension was kept for absorption measurement in a UV-vis spectrophotometer. Decrease in the maximum absorption of MB at 664 nm with irradiation time indicates decomposition of MB. We also performed photocatalytic test for blank MB solution without any catalyst for same irradiation time of 90 min. The degradation (D) of MB can be calculated by using following the equation:
 | (1) |
2.4 Photocatalyst reusability test
The stability of g-C3N4 as a photocatalyst was tested by repeating the photocatalytic process of recovered photocatalyst. After the initial photocatalytic reaction, the centrifuged product of the catalyst (as mentioned in photocatalytic activity study above) was recovered and dried at 50 °C, and then re-dispersed in fresh MB solution. The photocatalytic reaction was started and after each cyclic run the catalyst was recovered, dipped in fresh MB solution and irradiated for 90 min. The concentration change of MB was measured with UV-vis spectroscopy at its ∼664 nm absorption peak. The process was repeated for 3 times. We tested XRD pattern of the catalyst after the 3rd photocatalytic run and compared it with that of the pure catalyst to observe any degradation in the catalyst quality after catalytic reaction.2.5 Radical trapping experiment
Presence of active radical species and their role on photocatalysis was tested by trapping the active radicals by using some sacrificial agents. Ammonium oxalate (AO), tert-butanol (t-BA) and p-benzoquinone (BQ) were used as hole (h+), hydroxyl radical scavenger (˙OH) and superoxide radical (O2˙−) scavenger, respectively. The experimental procedure involves addition of 1 mM of scavengers to catalyst–dye solution (30 mg, 8 mg L−1) in 100 mL beaker. For each of the quenchers (tert-BA, AO and BQ) three experiments were conducted. The MB-catalyst-quencher was then exposed to visible light for different irradiation time, and the changes in the concentration of MB were monitored at 664 nm in a UV-vis spectrophotometer.3. Results and discussion
3.1 Structural studies
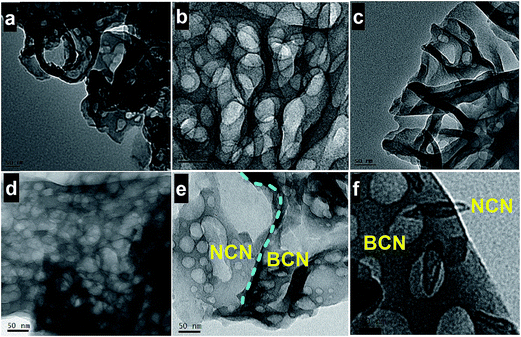
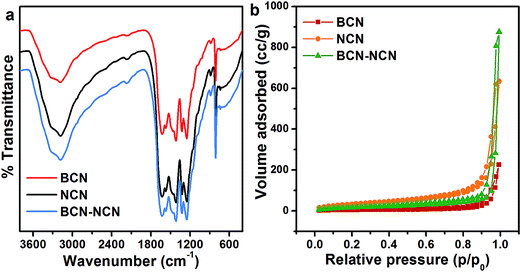
XRD pattern of BCN, NCN and BCN–NCN are shown in Fig. 1. The samples display an intense (002) diffraction peak at 2θ = 27.25°. This peak corresponds to interlayer stacking of aromatic CN unit with d = 3.27 Å.4,8 Interestingly, the enhancement of (002) peak intensity in NCN demonstrates an improvement in crystallinity after liquid exfoliation of BCN. There is a slight shift in (002) peak position from 27.25° in BCN to 27.74° in NCN, with a corresponding lowering of stacking distance from d = 3.27 Å to d = 3.21 Å. There is, however, a lowering in (002) peak intensity in BCN–NCN with a corresponding d-spacing of 3.23 Å. The lowering of peak intensity is possibly due to the conjugation of two systems (BCN and NCN) with different degree of crystallinity. All the samples display another low intensity diffraction peak at 2θ = 13.5°, corresponding to in-plane ordered tri-s-triazine (s-heptazine) units having crystalline plane (100).3,4,8 As observed from Fig. 1, there is no obvious change in the intensity or position of this peak in NCN and in BCN–NCN. For the (100) peak, the interlayer spacing of d = 6.75 Å specifies hole-to-hole distance of the nitride pores in g-C3N4 or intraplanar size of tri-s-triazine unit.3 The basic unit structure of graphitic g-C3N4 is shown in inset of Fig. 1.Morphological features of BCN, NCN and BCN–NCN are shown in Fig. 2. TEM images of BCN (Fig. 2a) show thick layered structures, which seems to get thinner in NCN with porous structures (Fig. 2b). The honeycomb like porous structures are formed by the release of NH3 and CO2 gases during condensation of urea. Initially soft bubbles are formed on calcination, indicating the starting of the release of these gases from urea. These bubbles finally burst out and forms porous structures of C3N4 on condensation of urea.21 TEM images of NCN obtained at different locations show thick and thin region of the layers (Fig. 2c). As evident from Fig. 2d, there are overlapping layers of BCN and NCN in the heterostructure constituting of bulk (BCN) and nanosheets (NCN) of g-C3N4. Fig. 2e shows another TEM image taken at a different location of the BCN–NCN sample. The image contains two regions, and we suppose that the thick BCN layers covers up few portion of thin NCN layers. The high resolution image taken on another location shows some folded structure and porous sheet (see Fig. 2f). Formation of paper-fold structure in the nanosheets of g-C3N4 has been reported by Dong et al.22 Chemical structures of BCN, NCN and BCN–NCN are further investigated with FTIR (see Fig. 3a). The samples show an intense absorption band at 808 cm−1 corresponding to breathing mode of aromatic ring of carbon nitride.23 Absorption bands at 1200–1600 cm−1 are assigned to typical symmetric stretching, asymmetric vibrations of C–N–C and C–NH–C units in aromatic ring.12,13,22 The broad absorption bands at 3000–3400 cm−1 are attributed to uncondensed primary amine (–NH2) or an imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH) and absorbed hydroxyl groups.23 Fig. 3b shows the N2 adsorption–desorption isotherm of the samples. The surface area of BCN, NCN and BCN–NCN determined from the isotherm by multipoint BET method are 18 m2 g−1, 121 m2 g−1 and 62 m2 g−1, respectively.
NH) and absorbed hydroxyl groups.23 Fig. 3b shows the N2 adsorption–desorption isotherm of the samples. The surface area of BCN, NCN and BCN–NCN determined from the isotherm by multipoint BET method are 18 m2 g−1, 121 m2 g−1 and 62 m2 g−1, respectively.
3.2 Optical studies
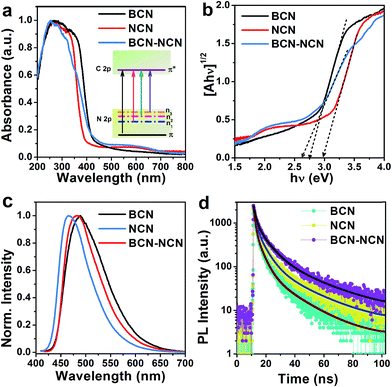
Changes in optical properties of bulk, nanosheets and heterostructure of g-C3N4 are investigated with UV-vis absorption spectroscopy. As depicted in Fig. 4, in comparison to BCN the absorption edge is blue shifted in NCN. The UV absorption edge of BCN–NCN is slightly extended to the visible region. Interestingly, NCN and BCN–NCN contain additional absorption in visible region (between 450–700 nm) which is otherwise absent in BCN. The maximum absorption in the ultraviolet (UV) region involves π → π* electronic transition, and the second narrow absorption in UV is due to n → π* transition.24,25 This n → π* transition is found to be responsible for the visible absorption at 450–700 nm. Several such n → π* transitions are possible in g-C3N4 involving N 2p lone pair orbital (n), and these transitions favor distortion of planarity of s-heptazine ring of g-C3N4.14,26 Chen et al. have discussed the redistribution of electron density resulting from the distortion of heptazine ring, and the distortion of the ring favor several n–π* optical transitions (shown inside in Fig. 4a).14 The band gap of each sample is determined by plotting (Ahν)1/2 vs. hν, where A is the absorbance. The plotted graph is shown in Fig. 4b and the resulting band gap values are presented in Table 1. The indirect band gap of BCN (2.74 eV) is enlarged to 2.97 eV in NCN. In NCN the stacking distance of the layers decreases and the layers are densely packed. Considering this, the enlargement in band gap in NCN can be attributed to quantum confinement of electrons.13 The heterostructure BCN–NCN has an effective band gap of 2.62 eV, and the observed effective band gap could be due to the band edge shift caused by the electronic coupling of BCN and NCN. We speculate that different extent of electronic coupling between BCN and NCN at the interface in the heterostructure and possible band bending at the heterostructure interface could have resulted in the observed reduction in the effective band gap of BCN–NCN. PL spectra of BCN, NCN and BNC-NCN are displayed in Fig. 4c. BCN displays a single broad emission peak at 490 nm. This peak is blue shifted to 465 nm in NCN. BCN–NCN has an emission peak at 483 nm, which is near the emission peak of BCN. These emissions could be assigned to π* → n transition.24,25,27 The shift in the peak position results from the change in the packing of the layered structures in the samples that allows electron–hole recombination of π* electrons with holes in the n orbitals (e.g. n1, n2, n3 etc.), which is in conformity with the results of UV-vis spectroscopy. Merschjann et al. attributed the π* → n emission to molecular exciton generated in the s-heptazine ring.28 The efficiency of charge carrier recombination giving rise to the excitonic emission would possibly be different in BCN, NCN and BCN–NCN. To understand the carrier recombination dynamics, we performed time resolved photoluminescence (TRPL) measurements of the samples. Fig. 4d shows the TRPL curves for different samples. It is found that the tri-exponential fitting can best fit the experimental decay curves and the decay components (τ1, τ2, τ3) and relative amplitudes of the decay species (A1, A2, A3) are shown in Table 1. As it is seen in Table 1, first decay component (τ1) is prolonged in NCN and BCN–NCN as compared to that in BCN, but the relative percentage of these species are lower than BCN. Consideration of second decay component (τ2) of carrier lifetime with their relative abundance reveal that the corresponding value increases in NCN and BCN–NCN as compared to BCN. Impressively, the third component of lifetime (τ3) and percentage amplitude of this component (A3) increases from 18.8 ns (19%) in BCN to 23.3 ns (25%) and 26.8 ns (32%) in NCN and BCN–NCN, respectively. The average lifetime (tav), determined using the formula , increases from 13.2 ns in BCN to 17.4 ns in NCN and raises to 21 ns in BCN–NCN. The different decay components of carrier lifetime and their relative abundance in the three samples could be linked with bulk or thin layered structures as well as presence of buckling sites, presence of terminal groups and localized states linked with nitrogen or carbon related defects.13,25,27,29 The longest decay components, its high abundance and the longest average lifetime in BCN–NCN could be associated with the interface mediated carrier separation process in the heterostructure formed between BCN–NCN. Possibly the band bending at the interface of the heterostructure formed between BCN and NCN provides the driving force for the efficient carrier separation and long carrier lifetime.
, increases from 13.2 ns in BCN to 17.4 ns in NCN and raises to 21 ns in BCN–NCN. The different decay components of carrier lifetime and their relative abundance in the three samples could be linked with bulk or thin layered structures as well as presence of buckling sites, presence of terminal groups and localized states linked with nitrogen or carbon related defects.13,25,27,29 The longest decay components, its high abundance and the longest average lifetime in BCN–NCN could be associated with the interface mediated carrier separation process in the heterostructure formed between BCN–NCN. Possibly the band bending at the interface of the heterostructure formed between BCN and NCN provides the driving force for the efficient carrier separation and long carrier lifetime.
| Sample | Band gap (eV) | Lifetime of carriers | tav (ns) | ||
|---|---|---|---|---|---|
| τ1 (ns) (A1%) | τ2 (ns) (A2%) | τ3 (ns) (A3%) | |||
| BCN | 2.74 | 1.03 (43) | 4.3 (38) | 18.8 (19) | 13.2 |
| NCN | 2.97 | 1.34 (33) | 5.5 (42) | 23.3 (25) | 17.4 |
| BCN–NCN | 2.62 | 1.60 (27) | 7.1 (41) | 26.8 (32) | 21.0 |
3.3 Photocatalysis study
The photocatalytic activities of BCN, NCN and BCN–NCN are evaluated by monitoring the changes in the maximum absorption at 664 nm for an aqueous MB solution under visible light. Fig. 5a shows the absorption spectra of MB as blank solution as well as in presence of catalyst BCN–NCN. Before irradiation, both blank and catalyst loaded MB solution are stirred in dark for 45 min for adsorption–desorption equilibration. When light is turned off (dark reaction) and the absorption of MB is measured, blank MB shows only negligible decrease in initial concentration, whereas MB solution in presence of BCN–NCN displays a substantial decrease in initial concentration (Fig. 5b). After turning on the light source and subjecting the solution to visible light irradiation, a remarkable decrease in concentration of MB solution is observed for BCN–NCN as compared to BCN or NCN. Impressively, the direct decomposition of MB in absence of photocatalyst is not detected. The decrease in initial concentration of MB in each case is shown in Fig. 5b. The trend in the photocatalytic activities follows the trend: BCN–NCN > NCN > BCN. Fig. 5c shows the linear relationship of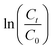 vs. irradiation time (t), and the calculated values of rate constants are shown in Fig. 5d. It can be seen from Fig. 5d, about 92% of MB is photodegraded by BCN–NCN over irradiation for 90 min with associated rate constant (k) of 0.037 min−1, while 83% is photodegraded by NCN for the same irradiation time with rate constant of 0.020 min−1. MB removal over BCN is 54% with the lowest k value of 0.010 min−1. Electron collection in g-C3N4 may have taken place by two pathways: self-sensitization of MB under visible light in which photoexcited electrons in MB are transferred to CB of g-C3N4, and the other being direct excitation of electrons to CB of C3N4 under visible light.30 Wide band gap semiconductor, such as TiO2, which does not absorb visible light, self-sensitized decomposition of organic dyes under visible light is prominent. However, g-C3N4 has band gap that lies in the visible region of solar spectrum. Therefore, direct photoexcitation of electrons to CB in g-C3N4 is feasible, which further supports the fact that g-C3N4 mediated photodegradation of MB is dominating rather than self-sensitized degradation of MB. From the results, we attempt to provide some explanations for the observed differences in the photocatalytic activity of BCN, NCN and BCN–NCN in the degradation of MB. We will try to explain the possible pathways that could provide an idea of the differences in the photodegradation of MB in each of the samples. In case of BCN, as it has bulk structure, presence of large numbers of stacked layers could be expected, and the photoexcited carriers might undergo facile bulk recombination before reaching to the surface. Those carriers which could migrate to the catalyst surface could interact with less numbers of adsorbed MB dye molecule (because of the least surface area in BCN). Therefore, a reduction in photocatalysis is expected. On the other hand, NCN has a sufficiently large surface area and the numbers of available surface carriers are expected to be high on the surface because of the lowering of bulk recombination. Therefore, a large percentage of freely available carriers are available on the surface to interact with sufficient numbers of adsorbed MB molecule. Even though the surface area of BCN–NCN is lower than that of NCN, the high photocatalytic activity in BCN–NCN can be associated with its visible absorbing band gap and mostly due to the sufficiently prolonged carrier lifetime mediated by heterostructure interface.
vs. irradiation time (t), and the calculated values of rate constants are shown in Fig. 5d. It can be seen from Fig. 5d, about 92% of MB is photodegraded by BCN–NCN over irradiation for 90 min with associated rate constant (k) of 0.037 min−1, while 83% is photodegraded by NCN for the same irradiation time with rate constant of 0.020 min−1. MB removal over BCN is 54% with the lowest k value of 0.010 min−1. Electron collection in g-C3N4 may have taken place by two pathways: self-sensitization of MB under visible light in which photoexcited electrons in MB are transferred to CB of g-C3N4, and the other being direct excitation of electrons to CB of C3N4 under visible light.30 Wide band gap semiconductor, such as TiO2, which does not absorb visible light, self-sensitized decomposition of organic dyes under visible light is prominent. However, g-C3N4 has band gap that lies in the visible region of solar spectrum. Therefore, direct photoexcitation of electrons to CB in g-C3N4 is feasible, which further supports the fact that g-C3N4 mediated photodegradation of MB is dominating rather than self-sensitized degradation of MB. From the results, we attempt to provide some explanations for the observed differences in the photocatalytic activity of BCN, NCN and BCN–NCN in the degradation of MB. We will try to explain the possible pathways that could provide an idea of the differences in the photodegradation of MB in each of the samples. In case of BCN, as it has bulk structure, presence of large numbers of stacked layers could be expected, and the photoexcited carriers might undergo facile bulk recombination before reaching to the surface. Those carriers which could migrate to the catalyst surface could interact with less numbers of adsorbed MB dye molecule (because of the least surface area in BCN). Therefore, a reduction in photocatalysis is expected. On the other hand, NCN has a sufficiently large surface area and the numbers of available surface carriers are expected to be high on the surface because of the lowering of bulk recombination. Therefore, a large percentage of freely available carriers are available on the surface to interact with sufficient numbers of adsorbed MB molecule. Even though the surface area of BCN–NCN is lower than that of NCN, the high photocatalytic activity in BCN–NCN can be associated with its visible absorbing band gap and mostly due to the sufficiently prolonged carrier lifetime mediated by heterostructure interface.
Stability of photocatalyst is of paramount importance when practical applications of photocatalysts are concerned. Stability study was performed by recycling of photocatalytic degradation of MB on the catalysts surface for three times under visible light. We performed stability test for BCN–NCN as it exhibited highest photocatalytic activity. As Fig. 6a shows, there is no obvious photoactivity loss of MB after 3rd cycle. XRD pattern of the sample of un-irradiated BCN–NCN and that of the irradiated sample recovered after 3rd photocatalytic run is recorded, and as Fig. 6b clearly demonstrates there is no significant change in the diffraction pattern of BCN–NCN after the stability test. The small change in intensity of (002) peak can be ascribed to the presence of adsorbed MB molecule on C3N4 and π–π interaction between them, which after 3rd cyclic test minutely affects its crystallinity, confirming that BCN–NCN is quite a stable photocatalyst in terms of its practical applications. High photocatalytic activity of BCN–NCN may be caused by the presence of available carriers and formation of active radical species. Irradiation of MB–(BCN–NCN) solution under visible light will excite electrons from N 2p to C 2p with electrons on conduction band (CB) and holes in valence band (VB). Since the catalyst is dispersed in aqueous MB solution, the CB electrons would be able to reduce O2 adsorbed on C3N4 surface to form superoxide radical (O2˙−). The photogenerated holes in VB, however, are expected to form hydroxyl radical (˙OH) by reacting with H2O. For confirmation of the reactivity of different radicals and carriers on the photocatalysis, we used trapping experiments (TE) by adding few radical scavengers (SV) in the MB solution catalyzed by the stable photocatalyst BCN–NCN. For the experiment, tert-butanol (t-BA) is used as hydroxyl scavenger (˙OH), ammonium oxalate (AO) and p-benzoquinone (BQ) for scavenging hole (h+) and superoxide radical (O2˙−), respectively.31–33 It is seen in Fig. 7a that there is slight reduction in the degradation due to the addition of t-BA, indicating participation of less numbers of ˙OH on MB degradation. Photodegradation is slightly suppressed on adding AO to the MB solution, implying that holes play an active role in the MB decomposition. There is a dramatic decrease in MB removal on adding BQ in the solution, suggesting that O2˙− is the most active radical species leading to the degradation of MB. The results are quite reflected in Fig. 7b. In absence of any scavengers (SV), MB degradation by BCN–NCN is 92%, while on adding BQ as O2˙− scavengers the degradation of MB drops down to 31%.
3.4 Mechanism of photocatalytic degradation
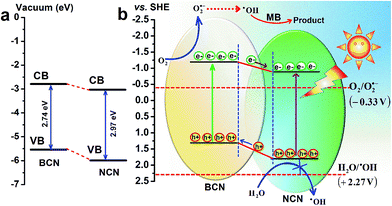
The band edge positions (VB and CB) of BCN–NCN are of much significance to understand the interface formation, band bending and the mechanism of chare carrier separation, as well as formation of O2˙− and ˙OH radicals. There are several reports on the band edge potential (VB and CB) of g-C3N4 in bulk and nanosheets, which is based on the results of electron spectroscopy measurement of potential with respect to SHE (standard hydrogen electrode), and theoretical calculations.34–38 These reports have speculated that on modification of bulk structure of C3N4 to few layered nanosheets the structure gets distorted, resulting in relative shifting in CB and VB edge position. For bulk and nanosheets, the CB edge potentials are found to be in between (−0.78 to −1.49 V) and VB between (1.3 to 1.86 V). Yang et al. observed a downward shifting of CB edge potential from −1.49 V in bulk to −1.40 V in nanosheets of g-C3N4.12 Li et al. observed a downward shifting of CB edge potential from −1.23 V to −0.88 V and VB edge shifting from 1.31 V to 1.86 V in bulk and nanosheets, respectively.36 Based on these results, we have provided a schematic band diagram (Fig. 8a and b) to show the band edge positions BCN–NCN heterostructure with respect to vacuum level as well as with reference to standard hydrogen electrode (SHE).34–38 Fig. 8a depicts a schematic of the conduction band (CB) and valence band (VB) edge positions in the heterostructure for the corresponding band gap values of 2.74 eV and 2.97 eV for BCN and NCN, respectively. Fig. 8b shows the band diagram of heterostructure with reference to SHE and the associated redox potential for ˙OH/H2O and O2/O2˙−. Because of different band alignment of VB and CB in the heterostructure, the electrons can move from CB of BCN to CB of NCN. Similarly, holes can migrate from VB of NCN to VB of BCN. The field at the interface provides the driving force in the facile separation of charge carriers, which finally become available to interact with O2 or H2O to form O2˙− and ˙OH radicals. If we consider the CB edge potential in between the above mentioned values of potential, the CB edge potential is more positive than the standard redox potential of O2/O2˙− (−0.33 V vs. SHE). Therefore, superoxide radical can be easily formed in this process. Considering the VB edge potentials having the reported values, the position of holes in VB is at lower potential than the standard redox potential of ˙OH/H2O (+2.27 V).34–38Both trapping experiment and band edge potentials of electrons in CB confirm that O2˙− has major contribution in the photodegradation of MB. Superoxide radicals (O2˙−) are, however, unstable in aqueous solution and readily transform to ˙OH. Formation of ˙OH from superoxide radical (O2˙−) occurs by multiple oxygen reduction reaction as shown below.39
| O2 + e− → O2˙− |
| O2˙− + 2H+ + e− → H2O2 |
| H2O2 + e− → ˙OH + OH− |
The generated hydroxyl radicals have the propensity to react with most of the organic compounds by direct electron transfer, H abstraction, etc.
We now propose a mechanism for the degradation of MB by hydroxyl radical on the surface of BCN–NCN. It might be considered that the strong adsorption of MB on C3N4 could be due to strong π–π interaction between MB and C3N4 that strongly held the dye–solid together. Besides, MB is a cationic dye molecule and g-C3N4 has delocalized π electrons in the s-heptazine ring, and also contains terminal N atom with lone pair of electrons. Therefore, there is a possibility of strong cationic-anionic columbic interaction between MB with g-C3N4 nanosheet at the solid–dye interface. There are different stages of degradation of whole MB molecule. In MB, the terminal N–CH3 groups which have the lowest binding energy (B.E) of 70.8 kcal mol−1 is first attacked by ˙OH radical.40–43 In the second step ˙OH radicals can attack the C–S+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (B.E ∼ 76 kcal mol−1) and transform this to C–S(
C bond (B.E ∼ 76 kcal mol−1) and transform this to C–S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C.41 This transformation facilitates opening up of the central aromatic ring by cleavage of N–C and S–C bond. Finally the functional groups are detached and the aromatic ring is destroyed to form the final degradation products.
O)–C.41 This transformation facilitates opening up of the central aromatic ring by cleavage of N–C and S–C bond. Finally the functional groups are detached and the aromatic ring is destroyed to form the final degradation products.
4. Conclusion
In conclusion, bulk, nanosheets and isotype heterostructure composed of g-C3N4 exhibit tunable absorption and photoluminescence properties. Compared to bulk, nanosheets of C3N4 have sufficiently larger surface area with free charge carriers. Though the heterostructure shows lower specific surface area than that of the nanosheets, it has a reduced effective band gap and prolonged charge carriers lifetime. These modified forms of carbon nitride, viz. nanosheets and heterostructure, show improved photocatalytic activity in the degradation of MB under visible light. Significant improvement in the photocatalytic activity in the heterostructure is due to the suitably matching valence and conduction band levels that promote facile separation of photogenerated electrons and holes, making the carriers available for photochemical reaction. It is the photogenerated conduction band electrons in the heterostructure that facilitates the formation of active radical species ˙OH by oxygen reduction reaction, which finally interact with functional groups and aromatic ring of MB molecule and decompose it. The development of low cost heterostructure of g-C3N4 for efficient visible light photocatalysis will enable wide spread applications of g-C3N4 in various emerging applications.Acknowledgements
B. C. would like to thank IIT Guwahati for providing institute postdoctoral fellowship to carry out the postdoctoral research. The authors like to thank Central Instrument Facility for providing the in house characterization facilities.References
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- R. Li, H. Kobayashi, J. Guo and J. Fan, Chem. Commun., 2011, 47, 8584–8586 RSC.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- J. Xu, M. Shalom, F. Piersimoni, M. Antonietti, D. Neher and T. J. K. Brenner, Adv. Opt. Mater., 2015, 3, 913–917 CrossRef CAS.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. O. Muller, R. Schlogl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- A. Schwarzer, T. Saplinova and E. Kroke, Coord. Chem. Rev., 2013, 257, 2032–2062 CrossRef CAS.
- F. Su, S. C. Mathew, G. Lipner, X. Fu, M. Antonietti, S. Blechert and X. Wang, J. Am. Chem. Soc., 2010, 132, 16299 CrossRef CAS PubMed.
- A. Du, S. Sanvito and S. C. Smith, Phys. Rev. Lett., 2012, 108, 197207 CrossRef PubMed.
- F. Dong, Z. Wang, Y. Sun, W. K. Ho and H. Zhang, J. Colloid Interface Sci., 2013, 401, 70–79 CrossRef CAS PubMed.
- Y. Li, Y. Sun, F. Dong and W. K. Ho, J. Colloid Interface Sci., 2014, 436, 29–36 CrossRef CAS PubMed.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- P. Niu, L. Zhang, G. Liu and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- Y. Chen, B. Wang, S. Lin, Y. Zhang and X. Wang, J. Phys. Chem. C, 2014, 118, 29981–29989 CAS.
- Y. C. Lu, J. Chen, A. J. Wang, N. Bao, J. J. Feng, W. Wang and L. Shao, J. Mater. Chem. C, 2015, 3, 73–78 RSC.
- J. Zhou, M. Zhang and Y. Zhu, Phys. Chem. Chem. Phys., 2014, 16, 17627–17633 RSC.
- J. Zhang, M. Zhang, R. Q. Sun and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145 CrossRef CAS PubMed.
- F. Dong, Z. Li, P. Li and Z. Wu, New J. Chem., 2015, 39, 4737–4744 RSC.
- Z. Zhao, Y. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- X. Zhang, X. Xie, H. Wang, J. Zhang, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 18 CrossRef CAS PubMed.
- Z. Wang, W. Guan, Y. Sun, F. Dong, Y. Zhou and W. K. Ho, Nanoscale, 2015, 7, 2471–2479 RSC.
- F. Dong, Z. Zhao, T. Xiong, Z. Ni, W. Zhang, Y. Sun and W. K. Ho, ACS Appl. Mater. Interfaces, 2013, 5, 11392–11401 CAS.
- J. Xu, L. Zhang, R. Shi and Y. Zhu, J. Mater. Chem. A, 2013, 1, 14766–14772 CAS.
- Y. Li, J. Zhang, Q. Wang, Y. Jin, D. Huang, Q. Cui and G. Zou, J. Phys. Chem. B, 2010, 114, 9429–9434 CrossRef CAS PubMed.
- H. Zhang and A. Yu, J. Phys. Chem. C, 2014, 118, 11628–11635 Search PubMed.
- G. A. Meek, A. D. Baczewski, D. J. Little and B. G. Levine, J. Phys. Chem. C, 2014, 118, 4023–4032 CAS.
- J. Bian, J. Li, S. Kalytchuk, Y. Wang, Q. Li, T. Lau, T. A. Niehaus, A. L. Rogach and R. Q. Zhang, ChemPhysChem, 2015, 16, 954–959 CrossRef CAS PubMed.
- C. Merschjann, T. Tyborski, S. Orthmann, F. Yang, K. Schwarzburg, M. Lublow, M. Ch, L. Steiner and T. S. Niedrig, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 205204 CrossRef.
- P. Niu, G. Liu and H. M. Cheng, J. Phys. Chem. C, 2012, 116, 11013–11018 CAS.
- T. Lv, L. Pan, X. Liu, T. Lu, G. Zhu, Z. Sun and C. Q. Sun, Catal. Sci. Technol., 2012, 2, 754–758 CAS.
- Y. Yang, Y. Guo, F. Liu, X. Yuan, Y. Guo, S. Zhang, W. Guo and M. Huo, Appl. Catal., B, 2013, 142–143, 828–837 CrossRef CAS.
- F. T. Li, Y. Zhao, Q. Wang, X. J. Wang, Y. J. Hao, R. H. Liu and D. Zhao, J. Hazard. Mater., 2015, 283, 371–381 CrossRef CAS PubMed.
- Y. Sun and J. J. Pignatello, Environ. Sci. Technol., 1995, 29(8), 2065–2072 CrossRef CAS PubMed.
- S. Chu, Y. Wang, Y. Guo, J. Feng, C. Wang, W. Luo, X. Fan and Z. Zou, ACS Catal., 2013, 3, 912–919 CrossRef CAS.
- J. Xiao, Y. Xie, F. Nawaz, S. Jin, F. Duan, M. Li and H. Cao, Appl. Catal., B, 2016, 181, 420–428 CrossRef CAS.
- H. J. Li, B. W. Sun, L. Sui, D. J. Qian and M. Chen, Phys. Chem. Chem. Phys., 2015, 17, 3309–3315 RSC.
- H. Z. Wu, L. M. Liu and S. J. Zhao, Phys. Chem. Chem. Phys., 2014, 16, 3299–3304 RSC.
- X. Fan, L. Zhang, M. Wang, W. Huang, Y. Zhou, M. Li, R. Cheng and J. Shi, Appl. Catal., B, 2016, 182, 68–73 CrossRef CAS.
- G. Xin and Y. Meng, J. Chem., 2013, 2013, 1–5 Search PubMed.
- Q. Wang, S. Tian and P. Ning, Ind. Eng. Chem. Res., 2014, 53, 643–649 CrossRef CAS.
- F. Huang, L. Chen, H. Wang and Z. Yan, Chem. Eng. J., 2010, 162, 250–256 CrossRef CAS.
- H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard and J. M. Herrmann, Appl. Catal., B, 2002, 39, 75–90 CrossRef CAS.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J. M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |