Complexation and intercalation modes: a novel interaction of DNA and graphene quantum dots†
Liping Lu*,
Linqing Guo,
Xiayan Wang*,
Tianfang Kang and
Shuiyuan Cheng
College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: lipinglu@bjut.edu.cn; xiayanwang@bjut.edu.cn
First published on 29th March 2016
Abstract
The interaction of DNA and the large planar structures of graphene quantum dots is investigated by absorption spectroscopy, gel electrophoresis, circular dichroism, and melting temperature. Our study departs from previous reports of interactions only by π–π stacking interaction of DNA on the surface of graphene.
Graphene quantum dots (GQDs), a new class of zero-dimensional carbon quantum dots with lateral sizes less than 100 nm, have recently attracted tremendous amounts of attention for their calculated theoretical properties and diverse applications.1,2 GQDs are photoluminescent because of quantum confinement and edge effects (such as zigzag and armchair edges), and thus demonstrate great promise for potential applications in many technological fields such as nanocomposites, bio-imaging, and photochemical catalysis.3,4 Much attention has focused on the assembly applications of graphene/DNA hybrid materials.5 According to previous reports, single-stranded DNA (ssDNA) chains flatly lay on the surfaces of graphene and its derivatives via π–π stacking interactions to form graphene–ssDNA hybrid material. Conversely, double-stranded DNA will leave the surface of graphene.6,7
Graphene's planar heterocyclic structure is similar to phenanthroline and dipyridophenazine, both of which are used as ligands in metal complexes that can bind DNA. The inter-plane spacing of the conjugated base pairs of DNA is 3.4 angstroms, similar to the interplane spacing in graphite. Some small molecules can bind to DNA through intercalative, hydrophobic (groove-binding) and electrostatic interactions.8,9 A well-known example is the Ru(II)–polypyridyl system.10–13 Owing to a combination of easily constructed rigid structures spanning all three spatial dimensions and a diverse set of photophysical properties, ruthenium(II) complexes with polypyridine ligands are known as “DNA light-switch” molecules.14,15 The similarities between the atomic structure of the carbon framework of GQDs and the polypyridyl ligands in the well-known Ru(II)–polypyridyl systems led us to investigate the interaction of graphene quantum dots with DNA. It remains unclear if graphene quantum dots, with planar heterocyclic structures, can interact with DNA in the same manner as metal complexes.
In this paper, we report the interaction of GQDs with well-matched DNA (WM-DNA), abase DNA (AB-DNA) and amino-modified DNA (AM-DNA) studied by absorption spectroscopy, circular dichroism, polyacrimide gel electrophoresis, and melting temperature analysis (Scheme 1).
 | ||
| Scheme 1 The three binding modes of GQDs with different sequence of dsDNA, GQDs-WM-DNA (a); GQDs-AB-DNA (b); GQDs-AM-DNA (c). | ||
As revealed by high-resolution transmission electron microscopy (HR-TEM), the GQDs have an average lateral diameter of 5.0 nm with a narrow size distribution of 3–7 nm (Fig. S1b†). Interestingly, the sizes change bigger when GQDs interacted with DNA (Fig. S2†), which proved GQDs is really interacted with GQDs.
Topographic morphology images of a typical tapping-mode, an AFM image, were shown in Fig. S1d,† demonstrated that most of the GQDs consisted of few graphene layers with a particle size mainly within the narrow range of 1–2 nm (Fig. S1e†), and the resultant GQDs have narrow distribution and mostly single or double-layered GQDs.16–19
The intensity of fluorescence of the GQDs at 445 nm (under excitation at 380 nm) increased after incubation with three kinds of DNA (Fig. 1). Previous reports confirmed that the emission peak at 445 nm is attributed to electron–hole recombination or quantum size effect/zig-zag effect.20 The zigzag edge sites of quantum-sized GQDs are carbene-like with a triplet ground state. When the GQDs bind to the DNA, the GQDs surface is passivated and the fluorescence intensity of GQDs is efficiently enhanced.17,21–23 Because no change of the fluorescence intensity of graphene oxide (GO) was observed after GO was incubated in DNA (Fig. S3†), it is likely that GQDs interact with DNA like metal complexes. Fig. S4† displays the high-resolution C 1s peaks (XPS) from conditioned GQDs before and after DNA interaction. The presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, C
C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH bands confirmed that the presence groups in GQDs. Moreover, the C 1s atomic% of three complexes approximately equal to the sum of their two monomers, respectively. These results proved interaction between GQDs and DNAs do exit.
O and COOH bands confirmed that the presence groups in GQDs. Moreover, the C 1s atomic% of three complexes approximately equal to the sum of their two monomers, respectively. These results proved interaction between GQDs and DNAs do exit.
UV-vis absorption spectroscopy is often used to study DNA interactions with small molecules.9,24 Here we compared UV absorption spectra of the free GQDs and GQDs–DNA complexes (Fig. 2). The absorption spectrum of the GQDs exhibited the typical peaks at 206 nm and 230 nm which are assigned to a π–π* transition.18 The absorbance of GQDs at 206 nm and 230 nm with the WM-DNA addition took place hypochromism and bathochromism. These results imply that intercalation is the likely interaction mode. Intercalative modes involve a stacking interaction between the chromophore and the DNA base pairs.25 When GQDs intercalate into the bases pairs of DNA, there will be a stacking interaction between GQDs heterocycle and the base pair of DNA. The stacking interaction results in a decrease in the distance between intercalated GQDs and DNA bases. This decreased distance results in a corresponding decrease in this electronic interaction resulting in the observed hypochromic and bathochromic shifts.
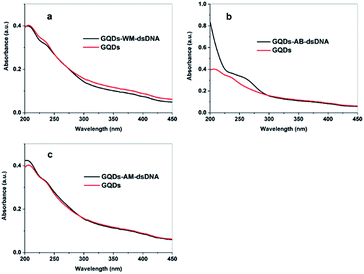 | ||
| Fig. 2 The UV-vis absorption spectrum of the GQDs in the absence or presence of WM-DNA (a), AB-DNA (b) or AM-DNA (c). | ||
Drastically different results were observed when the experiment was repeated with AM-DNA and AB-DNA. After addition of AM-DNA into and aqueous GQDs solution, the GQDs absorption bands at 206 nm and 230 nm were observed to shift to 205 nm and 229 nm respectively (Fig. 2(c)), and the absorption peak characteristic of DNA at 260 nm appeared. The observed hyperchromic shift might be indicative of groove binding.26 Here, the amino group likely facilitates the formation of CO–NH contacts with few of oxygen atoms present in the lattice of GQDs and binds by van der Waals interaction with DNA grooves and ring systems of GQDs connected by bonds with torsional freedom in order to adopt appropriate conformation that closely matches the helical turn of DNA grooves. The purine (adenine and guanine) and pyrimidine (cytosine and thymine) moieties are responsible for the electronic transitions observed at 260 nm. This presence of this absorption indicates that the GQDs interacts in groove of DNA and the conformation of DNA changes.
Additionally, the absorbance of GQDs at 206 nm, 230 nm with the AB-DNA addition took place hyperchromic (Fig. 2(b)). In this case we suggest that GQDs inserted into base pairs, because the corresponding changes of AB-DNA in its structure and conformation made GQDs insertion into the base pairs easier.27 When GQDs insert into the bases pairs of DNA, the base pair of DNA will be pushed out. The stacking interaction like as GQDs-WM-DNA could not appear. It resulted in hypochromic.28 The structural lability of AB-DNA facilitates the insertion of GQDs into the base pairs.
Polyacrylamide gel electrophoresis (PAGE) measurements were carried out to demonstrate the electrophoretic mobility of DNA and GQDs–DNA complexes (Fig. S5†). The differences in mobility of the materials demonstrate complexation forming.29 Mobility of a charged species in electrophoresis depends primarily on charge and molecular weight. The GQDs-WM-DNA band is behind the band of WM-DNA because GQDs bound to the WM-DNA and GQDs-WM-DNA are heavier than WM-DNA. GQDs bound to the AB-DNA and AM-DNA have also resulted in mobility decreases.30
Circular dichroism (CD) is a valuable tool for mapping conformational properties of particular DNA molecules. CD is a phenomenon originating from interactions of chiral molecules with circularly polarized electromagnetic rays. CD spectra of three kinds of DNA and their complexes with GQDs are included in Fig. 3. These spectra show the characteristic image of synthetic DNA as a positive long wavelength band at about 260–280 nm and negative bands at 210 nm and 245 nm.31
The CD spectrum of WM-DNA is very similar to that of the GQDs-WM-DNA, indicating that the GQDs did not influence the conformation and structure WM-DNA in any way (Fig. 3(a)). However, conformation of AB-DNA was changed after incubation with GQDs (Fig. 3(b)). The negative band at the 241 nm is blue shifted to 238 nm. The positive long wavelength band at 277 nm becomes higher indicating GQDs insertion into the base pairs and resulting change the conformation of AB-DNA.32 Meanwhile, CD of AM-DNA-GQDs has also differences indicating a change in conformation of DNA when GQDs bind in DNA groove (Fig. 3(c)).33
Finally, the melting temperatures of three kinds of DNA were measured in a 5 mM phosphate buffer solution (Fig. S6†). The results proved the interaction mode of between GQDs and WM-DNA, AM-DNA and AB-DNA are intercalation, groove binding and insertion, respectively. First, the Tm of GQDs-AB-DNA complexes was significantly lower than that abase-DNA. The AB-DNA melting temperature dropped from 42 degrees to 40 degrees than without GQDs, suggesting that the GQDs decreases the stability of AB-DNA. The insertion breaks the base pairs staking strength and results in the observed decrease in Tm. The Tm of AM-DNA-GQDs dropped from 38 degrees to 37 degrees indicating that the CO–NH bond weakens hydrogen-bonding of base pairs. Importantly, no change of the melting curves of WM-DNA and GQDs-WM-DNA were observed leading us to conclude that the GQDs do not influence the stability of WM-DNA and the interaction mode is intercalation.
Conclusions
In summary, we have successfully prepared three kinds of GQDs–DNA complexes and enunciated the binding modes of GQDs with single-base deleted DNA, NH2-labled DNA and well match DNA. The results showed that the GQDs could intercalate base pairs of WM-DNA like the ligands of metal complexes. It is expected that this novel strategy of GQDs–DNA complexation will open new opportunities for development of biosensor and promote the applications in nanobiotechnology.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21005005, 21375005, and 21475006), the Program for New Century Excellent Talents in University (NCET-12-0603) and Special Fund for Scientific Instruments (21527808).Notes and references
- X. Tan, Y. Li, X. Li, S. Zhou, L. Fan and S. Yang, Chem. Commun., 2015, 51, 2544–2546 RSC.
- Y. Zhu, G. Wang, H. Jiang, L. Chen and X. Zhang, Chem. Commun., 2015, 51, 948–951 RSC.
- H. Zhao, Y. Chang, M. Liu, S. Gao, H. Yu and X. Quan, Chem. Commun., 2013, 49, 234–236 RSC.
- D. Li, M. B. Mueller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- T. Premkumar and K. E. Geckeler, Prog. Polym. Sci., 2012, 37, 515–529 CrossRef CAS.
- L. Sun, N. Hu, J. Peng, L. Chen and J. Weng, Adv. Funct. Mater., 2014, 24, 6905–6913 CrossRef CAS.
- Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS PubMed.
- Z. Liu, M. Jiang, Y. Li, Z. Wu and J. Yang, Inorg. Chim. Acta, 2009, 362, 1253–1259 CrossRef CAS.
- M. Sirajuddin, S. Ali and A. Badshah, J. Photochem. Photobiol., B, 2013, 124, 1–19 CrossRef CAS PubMed.
- C. V. Kumar, J. K. Barton and N. J. Turro, J. Am. Chem. Soc., 1985, 107, 5518–5523 CrossRef CAS.
- J. Lecomte, A. Kirsch-De Mesmaeker, M. M. Feeney and J. M. Kelly, Inorg. Chem., 1995, 34, 6481–6491 CrossRef CAS.
- Y. Liu, T. Chen, Y. Wong, W. Mei, X. Huang, F. Yang, J. Liu and W. Zheng, Chem.-Biol. Interact., 2010, 183, 349–356 CrossRef CAS PubMed.
- S. Satyanarayana, J. C. Dabrowiak and J. B. Chaires, Biochemistry, 1992, 31, 9319–9324 CrossRef CAS PubMed.
- G. J. Ryan, F. E. Poynton, R. B. Elmes, M. Erby, D. C. Williams, S. J. Quinn and T. Gunnlaugsson, Dalton Trans., 2015, 44, 16332–16344 RSC.
- J. Liu, B. Ye, H. Li, Q. Zhen, L. Ji and Y. Fu, J. Inorg. Biochem., 1999, 76, 265–271 CrossRef CAS.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2011, 134, 15–18 CrossRef PubMed.
- J. Shen, Y. Zhu, C. Chen, X. Yang and C. Li, Chem. Commun., 2011, 47, 2580–2582 RSC.
- M. Zhang, L. Bai, W. Shang, W. Xie, H. Ma, Y. Fu, D. Fang, H. Sun, L. Fan and M. Han, J. Mater. Chem., 2012, 22, 7461–7467 RSC.
- D. Pan, L. Guo, J. Zhang, C. Xi, Q. Xue, H. Huang, J. Li, Z. Zhang, W. Yu and Z. Chen, J. Mater. Chem., 2012, 22, 3314–3318 RSC.
- S. Zhu, J. Zhang, S. Tang, C. Qiao, L. Wang, H. Wang, X. Liu, B. Li, Y. Li and W. Yu, Adv. Funct. Mater., 2012, 22, 4732–4740 CrossRef CAS.
- Y. Dong, G. Li, N. Zhou, R. Wang, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 8378–8382 CrossRef CAS PubMed.
- J. Shen, Y. Zhu, X. Yang, J. Zong, J. Zhang and C. Li, New J. Chem., 2012, 36, 97–101 RSC.
- Y. He, X. Wang, J. Sun, S. Jiao, H. Chen, F. Gao and L. Wang, Anal. Chim. Acta, 2014, 810, 71–78 CrossRef CAS PubMed.
- S. T. Saito, G. Silva, C. Pungartnik and M. Brendel, J. Photochem. Photobiol., B, 2012, 111, 59–63 CrossRef CAS PubMed.
- T. J. Bandy, A. Brewer, J. R. Burns, G. Marth, T. Nguyen and E. Stulz, Chem. Soc. Rev., 2011, 40, 138–148 RSC.
- A. M. Pyle, J. P. Rehmann, R. Meshoyrer, C. V. Kumar, N. J. Turro and J. K. Barton, J. Am. Chem. Soc., 1989, 111, 3051–3058 CrossRef CAS.
- C. Zhou, J. Zhao, Y. Wu, C. Yin and Y. Pin, J. Inorg. Biochem., 2007, 101, 10–18 CrossRef CAS PubMed.
- A. B. Pradhan, L. Haque, S. Bhuiya and S. Das, RSC Adv., 2015, 5, 10219–10230 RSC.
- A. Kulkarni, S. A. Patil and P. S. Badami, Eur. J. Med. Chem., 2009, 44, 2904–2912 CrossRef CAS PubMed.
- R. Rastogi, N. Dhindsa, C. R. Suri, B. D. Pant, S. K. Tripathi, I. Kaur and L. M. Bharadwaj, Mater. Chem. Phys., 2012, 135, 268–276 CrossRef CAS.
- J. Kypr, I. Kejnovská, D. Renčiuk and M. Vorlíčková, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS PubMed.
- P. U. Maheswari and M. Palaniandavar, J. Inorg. Biochem., 2004, 98, 219–230 CrossRef.
- Z. Li, Z. Zhu, W. Liu, Y. Zhou, B. Han, Y. Gao and Z. Tang, J. Am. Chem. Soc., 2012, 134, 3322–3325 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Data including the detailed procedure for experimental section. See DOI: 10.1039/c6ra00930a |
| This journal is © The Royal Society of Chemistry 2016 |


