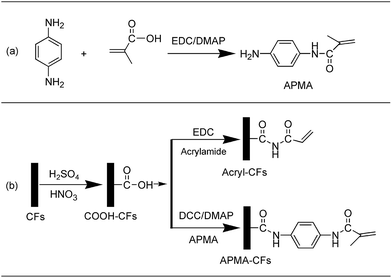
Improving the interfacial properties of carbon fibers/vinyl ester composites by vinyl functionalization on the carbon fiber surface
Xiuping Zhang,
Liu Liu,
Ming Li,
Yanjie Chang,
Lei Shang,
Jinglong Dong,
Linghan Xiao* and
Yuhui Ao*
College of Chemistry and Life Science, Jilin Province Key Laboratory of Carbon Fiber Development and Application, Changchun University of Technology, Changchun 130012, People's Republic of China. E-mail: aoyuhui@ccut.edu.cn; xiaolinghan1981@163.com; Fax: +86-431-85968078; Tel: +86-431-85968078
First published on 16th March 2016
Abstract
A novel “coupling agent” N-(4-amino-phenyl)-2-methyl-acrylamide (APMA) was synthesized to efficiently enhance interfacial interactions between the carbon fiber (CFs) and vinyl ester resin (VE). Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) were employed to characterize functional groups and chemical compositions of a carbon fiber surface, which indicated that the vinyl groups of APMA were grafted successfully on the surface of the CFs. Four different treatments of CFs (pristine CFs, carboxylic-CFs, acrylamide-CFs and APMA-CFs) were used to prepare the CF-reinforced VE composites. The mechanical properties were analysed and a micro-droplet test was performed to investigate the enhancement effect of the coupling agent on the interfacial strength of the composites. Scanning electron microscopy (SEM) showed that APMA-CFs were uniformly distributed throughout the matrix, and no open ring holes were found at the cross-section of the composites; furthermore, after micro-droplet test, some matrix was still attached to the debonded CF surface. As a result, APMA functionalized CFs improved the flexural strength of the APMA-CF/VE composites by 19.4%, and significantly improved the interfacial shear strength between APMA-CFs and VE by 90.53%.
1. Introduction
Owing to their superior strength-to-weight ratio, and excellent mechanical and thermal stability, fiber-reinforced composites have been increasingly used as structural materials in some specialised areas, such as aerospace and transportation.1,2 Among various types of fibers, carbon fibers are usually used as reinforced materials because they possess a low weight, high tensile strength, high stiffness, high temperature tolerance, high chemical resistance, and low thermal expansion.3–5 Vinyl ester resins are widely recognized as highly corrosion resistant, low curing shrinkage rate and low cost resin.6 The mechanical properties of the carbon fiber reinforced vinyl ester resin composites depend not only on the intrinsic characteristics of the carbon fiber and the resin, but also on their interfacial properties.7 It is widely believed that good interfacial adhesion properties are essential to ensure efficient load transfers from resin matrix to reinforcing fibers.8 However, fiber reinforced polymer composites often possess poor interfacial adhesion which significantly limits their practical application.9Carbon fibers without surface modification present highly stable, non-polar and smooth graphitic surface which makes it difficult to provide ideal interfacial bonding strength with resin matrix.10,11 Thus, extensive researches have been devoted to the surface treatment of CF in order to improve interfacial properties of CF-reinforced composites, such as sizing process,12 electrochemical method,13 plasma treatment,14,15 oxidation,16 coating,17 grafting.18 Ma et al. functionalized carbon fibers with branched polyethyleneimine as a coupling agent by supercritical methods to improving the interfacial properties of carbon fiber-reinforced epoxy composites.19 Frédéric et al. managed to graft thiol functionalities at carbon fiber surface in order to enhance the adhesion strength with an acrylate matrix photo-cured by ultraviolet light.20 They also coated CF with a maleic anhydride plasma polymer and functionalized with vinyl and thiol groups to tailor the interface interactions of CF–acrylate matrix composites.21 Wang et al. functionalized CF with phenyl amine groups via aryl diazonium reaction “on water” to improve their interfacial bonding with resin matrix.22 Liu et al. managed to graft different average molar mass of poly(ethylene glycol) onto the glass fiber surface to control the interphase properties of glass fiber/polypropylene composites.9
In this work, chemically functionalized CF/VE composites were manufactured by compression moulding forming. A novel “coupling agent” N-(4-amino-phenyl)-2-methyl-acrylamide (APMA) was synthesized to efficiently enhance the CF–VE interfacial interactions for the improvement of flexural strength and interfacial shear strength. In this functionalization process, grafting APMA onto the CF surface set up a good bridge between the CFs and VE resin, acting as a “coupling agent” (Fig. 1). In addition, effects of carbon fiber interfacial with or without different surface treatment on the micromorphology, dynamic mechanical properties, mechanical properties and interfacial shear strength of composites are carefully investigated to facilitate better understanding of the effect of chemical modifications.
2. Experimental
2.1. Materials
The carbon fibers used in this study were polyacrylonitrile (PAN)-based type manufactured by Toray Co. under the trade name of T300B. Vinyl ester resin 890 was purchased from Fuchen Co., Ltd, Shanghai, China. The carbon fiber fabric was knitted by automatic sampling loom (Evergreen, CCI TECH INC., Taiwan). The details of carbon fiber fabric are tabulated in Table 1. All other chemicals, such as cumene hydroperoxide, sulfuric acid (H2SO4), nitric acid (HNO3), potassium bromide (KBr), N,N′-dicyclohexylcarbodiimide (DCC), methacrylic acid, acetone, N-(3-dimethylaminopropyl-N-ethylcarbondiimide) hydrochloride (EDC), acrylamide (AAm), 4-dimethylaminopyridine (DMAP), dichloromethane (DCE), p-phenylenediamine, ethanol and tetrahydrofuran (THF) were purchased from Aladdin Co., Ltd, Shanghai, China and used without further purification.| Physical properties | Details |
|---|---|
| Weave | Plain |
| Count (per inch) | Wrap.12.5/fill.13.5 |
| Weight (g m−2) | 220 |
| Thickness (mm) | 0.25 |
| Single fiber diameter (μm) | ∼7 |
2.2. The synthesis of N-(4-amino-phenyl)-2-methyl-acrylamide
To a 100 mL flask were added p-phenylenediamine (1.1203 g, 0.01036 mol) and DCE (50 mL). EDC (2.1532 g, 0.01123 mol) and DMAP (0.2138 g, 0.001750 mol) were added separately into the solution. The mixture was stirred until EDC and DMAP were dissolved, after which methylacrylic acid (0.8816 g, 0.01024 mol) was added. After another 48 h of stirring at r.t. under N2 atmosphere, the reaction was quenched with 20 mL of saturated brine.23,24 The organic layer was separated and DCE was evaporated off leading to brown oil. Flash chromatography on silica gel (eluent: DCE/ethyl acetate = 2/1, Rf = 0.45) afforded the product which was dried at 50 °C for 24 h (0.9532 g, 53% yield). The synthesis of the APMA was shown in Fig. 2a. 1H NMR (400 MHz, DMSO, ppm) δ 9.33 (s, 1H), 7.27–7.25 (d, 2H), 6.50–6.50 (d, 2H), 5.71 (s, 1H), 5.40 (s, 1H), 4.85 (d, 2H), 3.29 (s, 1H), 2.50–2.50 (t, 3H), 1.92 (t, 3H).2.3. Grafting of the vinyl functionalities at the surface of the fiber
Before surface modification, the carbon fiber fabrics in the acetone solution reflux 24 h at 75 °C for removing sizing agent, and the carbon fiber fabrics were washed using deionized water, and dried at 50 °C for 24 h. The carbon fiber fabrics were treated by a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of concentrated H2SO4/HNO3 at 70 °C for 2 h.25 The modified fibers were thoroughly washed with deionized water, and then dried at 50 °C for 24 h to obtain COOH-CFs.6 After acid treatment, carbon fibers with carboxylic acid group were further functionalized by acrylamide/EDC/THF (weight ratio of acrylamide to EDC to CF is 1
1 (v/v) mixture of concentrated H2SO4/HNO3 at 70 °C for 2 h.25 The modified fibers were thoroughly washed with deionized water, and then dried at 50 °C for 24 h to obtain COOH-CFs.6 After acid treatment, carbon fibers with carboxylic acid group were further functionalized by acrylamide/EDC/THF (weight ratio of acrylamide to EDC to CF is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solution: to a beaker with THF were added 1
2) solution: to a beaker with THF were added 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of acrylamide and EDC separately. Carbon fibers were added into the mixture and the beaker was sealed with plastic film. Ultrasonic vibration for 48 h at r.t. afforded acryl-CFs. COOH-CFs was further treated by APMA/DCC/DMAP/THF (weight ratio of APMA to DCC to DMAP to CF is 6
1 ratio of acrylamide and EDC separately. Carbon fibers were added into the mixture and the beaker was sealed with plastic film. Ultrasonic vibration for 48 h at r.t. afforded acryl-CFs. COOH-CFs was further treated by APMA/DCC/DMAP/THF (weight ratio of APMA to DCC to DMAP to CF is 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) solution: to a beaker with THF were added DCC, DMAP and APMA separately. After the solids were dissolved, carbon fibers were added into the mixture and the beaker was sealed with plastic film. Ultrasonic vibration for 48 h at r.t. afforded APMA-CFs. The procedure for modification of carbon fibers was shown in Fig. 2b.
12) solution: to a beaker with THF were added DCC, DMAP and APMA separately. After the solids were dissolved, carbon fibers were added into the mixture and the beaker was sealed with plastic film. Ultrasonic vibration for 48 h at r.t. afforded APMA-CFs. The procedure for modification of carbon fibers was shown in Fig. 2b.
2.4. Preparation of composite materials
The carbon fibers reinforced vinyl resin composites were prepared by compression moulding forming. Samples were prepared using a hand lay-up method by impregnating carbon fiber plain weave fabric with resin (resin and cumene hydroperoxide were mixed in a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio), stacking and vacuum them. The preparation conditions and the entire curing process of the composite are shown in Fig. 3, gelatinized at 108 °C for 15 min, keep the pressure on 6 MPa, cured at 123 °C for 2 h, postcured at 145 °C for 1 h, program control cooling to prepared carbon fiber reinforced composites.
1 mass ratio), stacking and vacuum them. The preparation conditions and the entire curing process of the composite are shown in Fig. 3, gelatinized at 108 °C for 15 min, keep the pressure on 6 MPa, cured at 123 °C for 2 h, postcured at 145 °C for 1 h, program control cooling to prepared carbon fiber reinforced composites.
2.5. Characterization and instruments
The micromorphology of the samples was examined by using scanning electron microscopy (SEM) (JSM-6700F and 5600, JEOL, Japan). The presence of surface elements and the chemical composition was determined by using X-ray Photoelectron spectroscope (Thermo ESCALAB 250, Monochromatic X-ray source (hν = 14860.6 eV)). The Fourier transform infrared (FTIR) spectrum was recorded within the 400–4000 cm−1 region on a Bruker-IFS 66V/S spectrometer using KBr pellets. The 1H NMR spectra was taken on a BRUKER AVANCE III HD 400. The dynamic mechanical performance of the composites was studied using a Dynamic mechanical analysis (DMA 7, Perkin Elmer, USA). Rectangular shaped samples of 30 × 4 × 0.5 mm3 were mounted in a large tension clamp. The measurements were performed in the tensile mode at a heating rate of 3 °C min−1 and a deformation frequency of 1 Hz. The temperature was conducted from 40 to 240 °C. The tensile and flexural properties of the composites were measured with a Shimadzu AGS-XD 50 kN universal tester at room temperature and 50% relative humidity. Tensile and flexural specimens were employed according to ASTM D3039 and ASTM D7264 standards. All the specimens were conditioned for 24 h before the measurements. A minimum of five specimens of each sample were tested for statistical evaluation. The interfacial shear strength was tested by using interfacial evaluation equipment (FA-620, East Rong Corporation of Japan). Single fiber was strained and fixed on the metal framework, and the vinyl resin was uniformly dropped on carbon fiber to prepare micro-droplets. After that, the samples were cured as same as the conditions of preparing composites. The interfacial strength τ between the CF and resin can be calculated using following equation.26| τ = F/πdcflcf | (1) |
3. Results and discussion
3.1. Synthesis and structural characterization
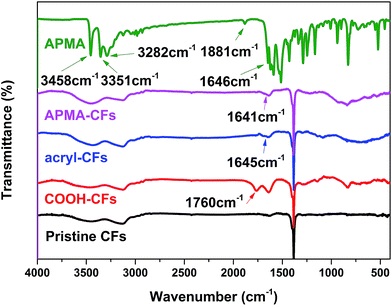
N-(4-Amino-phenyl)-2-methyl-acrylamide (APMA) was successfully synthesized by amidation reaction at room temperature between methacrylic acid and p-phenylenediamine (Fig. 2a). The compounds were characterized by spectroscopic methods and the detailed analysis data are shown in Fig. 4. 1H NMR spectrum of APMA showed a singlet for the equivalent methyl groups at 1.92 ppm. The chemical shift at 5.40 ppm and 5.71 ppm could be assigned to the vinyl units. The single signal at 9.33 ppm was assigned to the amino groups. Fig. 5 showed the IR spectrum of APMA. The absorption band around 3458 cm−1, 3351 cm−1 could be attributed to stretching vibration of N–H bond of aniline group. Another absorption appeared at 3282 cm−1 and 1646 cm−1 due to N–H bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of amide group, respectively. A weak absorption appeared at 1881 cm−1 might be an evidence of para-substituted benzene ring.
O bond of amide group, respectively. A weak absorption appeared at 1881 cm−1 might be an evidence of para-substituted benzene ring.
3.2. Fourier transform infrared (FTIR) analysis of functionalized CFs
FTIR tests were utilized to characterize modified carbon fibers (Fig. 5). As expected, after modification in acid solution for 2 h, a broad and intense absorption at 3150 cm−1 (O–H stretching vibration) and the bands at 1760 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from COOH groups situated at surface of carbon fibers) can be observed. The presence of different types of oxygen-containing functional groups demonstrated that the carbon fibers had been oxidized. The absorption at 1645 cm−1 and 1641 cm−1 may be attributed to the stretching vibrations of carbonyl groups in acryl-CFs and APMA-CFs, respectively. In addition, the sharp peaks at 3458 cm−1 and 3351 cm−1, stretching vibration absorption of N–H bonds of aniline in APMA completely disappeared in the spectrum of APMA-CFs. On the basis of these observations, it can be concluded that the acryl and APMA groups were successfully grafted onto the COOH-CFs, instead of physical absorbance on CFs.
O stretching vibrations from COOH groups situated at surface of carbon fibers) can be observed. The presence of different types of oxygen-containing functional groups demonstrated that the carbon fibers had been oxidized. The absorption at 1645 cm−1 and 1641 cm−1 may be attributed to the stretching vibrations of carbonyl groups in acryl-CFs and APMA-CFs, respectively. In addition, the sharp peaks at 3458 cm−1 and 3351 cm−1, stretching vibration absorption of N–H bonds of aniline in APMA completely disappeared in the spectrum of APMA-CFs. On the basis of these observations, it can be concluded that the acryl and APMA groups were successfully grafted onto the COOH-CFs, instead of physical absorbance on CFs.
3.3. The morphological observations of carbon fiber
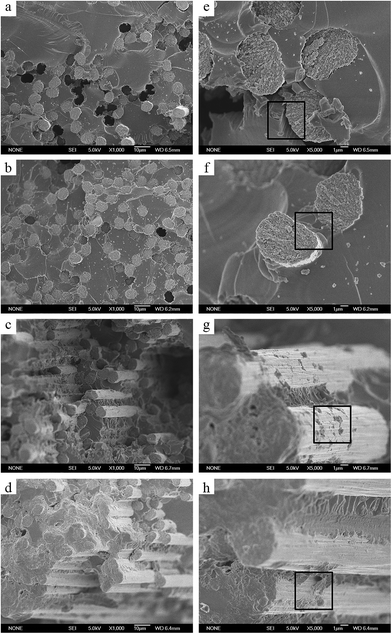
The surface morphologies of four different treatments of carbon fibers were examined by SEM; the images in the right column are the detail with enlarged scale of the images in the left column. As shown in Fig. 6a and e, the pristine CFs presented a rough surface, and a lot of narrow grooves parallel distributed along the longitudinal direction of the fiber. After oxidation reaction, a few of “black flecks” appeared on the COOH-CFs surface (Fig. 6b and f). However, as shown in Fig. 6g and h, acryl-CFs and APMA-CFs presented the differences of the surface morphologies after grafting vinylic functional groups. Some “granules” are uniformly distributed on the surface of the CFs, which could improve the fiber surface roughness so that improved interfacial interactions between CFs and matrix.22 Moreover, it can also prove that vinylic functional groups are grafted onto the CFs surface successfully. | ||
| Fig. 6 The SEM images of the pristine CFs (a and e), COOH-CFs (b and f), acryl-CFs (c and g) and APMA-CFs (d and h), respectively. | ||
3.4. XPS measurements
The XPS was performed to determine the chemical compositions of the CFs surface.25 Before carrying out XPS tests, acryl-CFs and APMA-CFs were sonicated at deionized water to get rid of the presence of any small molecules on CFs attached by other means such as van der Waals attraction force. The pristine CFs surface was composed mainly of carbon, oxygen and a small amount of nitrogen.27 The XPS survey spectra of the pristine CFs, COOH-CFs, acryl-CFs and APMA-CFs were shown in Fig. 7. The COOH-CFs showed the oxygen content increased to 22.81% compared with pristine CFs (21.22%) after treatment by mixed acid. This increased intensity in the oxygen elements was due to the oxygen-containing functional groups, which confirmed the successful acidification of the CFs. The contents of nitrogen of acryl-CFs and APMA-CFs, was 1.34% and 4.7%, which is higher than that of pristine CFs (0.24%). This increased N1s peak intensity was due to the amine functional groups of acrylamide and N-(4-amino-phenyl)-2-methyl-acrylamide reacted with the COOH functional groups of COOH-CFs, and then grafted onto the surface of the CFs. It was confirmed that the successful modification of acryl-CFs and APMA-CFs.A detailed analysis of the XPS spectra emphasized that CFs are successfully modified. The C1s peaks of pristine CFs were resolved into three peaks, and the three major peaks of the pristine CFs appeared at 284.6 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 286.0 eV (C–C), and 286.6 eV (C–O), which were attributed to the fiber structure of amorphous carbon. The C1s high-resolution spectrum results of COOH-CFs (Fig. 7f) showed one new binding energy peaks at 288.9 eV, which were attributed to O–C
C), 286.0 eV (C–C), and 286.6 eV (C–O), which were attributed to the fiber structure of amorphous carbon. The C1s high-resolution spectrum results of COOH-CFs (Fig. 7f) showed one new binding energy peaks at 288.9 eV, which were attributed to O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The functional groups of CFs surface could provide possibilities for further chemical treatment. The XPS (Fig. 7g) results showed that an additional peak appeared at 287.3 eV and 288.9 eV for the acryl-CFs, which were attributed to the O
O. The functional groups of CFs surface could provide possibilities for further chemical treatment. The XPS (Fig. 7g) results showed that an additional peak appeared at 287.3 eV and 288.9 eV for the acryl-CFs, which were attributed to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N bonds and C
C–N bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The XPS (Fig. 7h) results of APMA-CFs showed that an additional peak appeared at 287.3 eV and 288.9 eV for the APMA-CFs, which were attributed to the O
O. The XPS (Fig. 7h) results of APMA-CFs showed that an additional peak appeared at 287.3 eV and 288.9 eV for the APMA-CFs, which were attributed to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N and C
C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. The N–C
O bonds. The N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak certifies that the amide monomer attached onto the COOH-CFs surface by the nucleophilic attack of amine groups on the COOH groups of COOH-CFs, which suggesting that vinyl was grafted onto the surface of carbon fiber. The chemical reaction between –COOH and –NH2 was confirmed by tracing N1s, as shown in Fig. 7i and j.
O peak certifies that the amide monomer attached onto the COOH-CFs surface by the nucleophilic attack of amine groups on the COOH groups of COOH-CFs, which suggesting that vinyl was grafted onto the surface of carbon fiber. The chemical reaction between –COOH and –NH2 was confirmed by tracing N1s, as shown in Fig. 7i and j.
3.5. The SEM images of the composites cross-section
The carbon fibers reinforced vinyl resin composites were prepared by compression moulding forming. The entire curing process of the composite was shown in Fig. 3. During curing, free radical copolymerization not only occurred among vinyl ester resins but also between the vinyl groups of carbon fiber (CF) surface and the vinyl groups of vinyl ester resin (VE), initiated by cumene hydroperoxide. Formation of covalent bond between CFs and VE had been proven as an efficient method to enhance the interfacial adhesion of CFs. The protocol of forming covalent bonding at the interface of CF–VE matrix was illustrated in Fig. 1.In order to assess the morphology and interfacial adhesion of the prepared composites, the cross-sections of specimens were examined by SEM as shown in Fig. 8. Fig. 8a and e showed the microstructure of composites reinforced with pristine CFs. It can be seen that the VE matrix and the carbon fibers were separated from each other and some holes presented by pulling out of CFs from the matrix. It was attributed to the chemical inactivity of unmodified fibers, which resulted in weak interactions between matrix and untreated carbon fiber surfaces. In Fig. 8b and f, the fractured surface of sample showed less fiber pull-out compared with the VE/pristine CFs composites. The change of fractured surface morphology after CFs oxidative treatment led to the increase of specific surface area and the content of oxygen functional groups, which had a positive effect on the strength of the interfacial interaction. However, it was worth noting that in the Fig. 8c, d, g and h, the CFs were uniformly distributed through the matrix, and no open ring holes were found around them, indicative of the formation of covalent bonds between CFs and VE matrix enhanced the fiber–matrix interfacial adhesion. An amount of VE resin adhering to the carbon fiber surface could be seen in the Fig. 8g and h, which is attributed to the vinyl functional groups of carbon fibers reaction to vinyl resin.
3.6. Dynamic mechanical analysis
The viscoelastic properties (storage modulus and damping ratio) were measured by DMA, which provides information about the stiffness of the composites and the strength of the CFs–VE matrix interactions. The temperature dependence of the storage modulus (E′) at a frequency of 1 Hz was shown in Fig. 9a. The modulus of VE resin at 45 °C was 2.55 GPa. The introduction of the carbon fiber resulted in a 5.8-fold improvement in modulus, indicating effective stress transfer from the matrix to this reinforcement. Regarding the different composites, remarkably larger E′ increments are obtained for CFs treated with different methods. The E′ of the COOH-CFs/VE, acryl-CFs/VE and APMA-CFs/VE composites were 12.9%, 14.6% and 18.5% higher than that of pristine CFs/VE composites, respectively. These results are in very good agreement with those obtained from SEM analysis.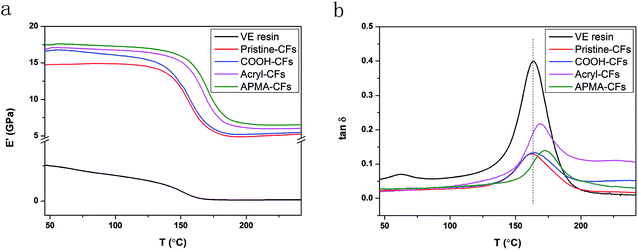 | ||
Fig. 9 Evolution of the storage modulus E′ (a) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (b) as a function of temperature, at the frequency of 1 Hz, for VE matrix and their composites. δ (b) as a function of temperature, at the frequency of 1 Hz, for VE matrix and their composites. | ||
The evolution of the damping ratio (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) as a function of temperature was shown in Fig. 9b. The Tg of pristine CFs/VE and COOH-CFs/VE composites were consistent with the Tg of VE resin (about 163.7 °C). However, the Tg of the acryl-CFs/VE and APMA-CFs/VE composites were higher than that of VE resin, which were 169.1 °C and 173.1 °C, respectively. It can be explained that the increase of the glass temperature arouse from the formation of new chemical bond between carbon fiber and vinyl resin. The reasons proposed to explain the improvement in the glass transition temperature of the vinyl-CFs/VE composites could be as follows: the vinyl-functionalized COOH-CFs with vinyl functional groups can maintain the compatibility of CFs within the VE matrix, which formed a 3D network structure throughout the whole VE matrix and the vinyl functionalized surface of the CFs can provide reaction sites for free-radical polymerization; the sites can construct a covalently bonded interphase structure between the vinyl-functionalized CFs and VE, which resulted in a strong interfacial interaction and restricted the movement of resin segments.6 Possessing relative rigid phenyl group in APMA-CFs could be one of reasons for higher Tg than acryl-CFs/VE composites.
δ) as a function of temperature was shown in Fig. 9b. The Tg of pristine CFs/VE and COOH-CFs/VE composites were consistent with the Tg of VE resin (about 163.7 °C). However, the Tg of the acryl-CFs/VE and APMA-CFs/VE composites were higher than that of VE resin, which were 169.1 °C and 173.1 °C, respectively. It can be explained that the increase of the glass temperature arouse from the formation of new chemical bond between carbon fiber and vinyl resin. The reasons proposed to explain the improvement in the glass transition temperature of the vinyl-CFs/VE composites could be as follows: the vinyl-functionalized COOH-CFs with vinyl functional groups can maintain the compatibility of CFs within the VE matrix, which formed a 3D network structure throughout the whole VE matrix and the vinyl functionalized surface of the CFs can provide reaction sites for free-radical polymerization; the sites can construct a covalently bonded interphase structure between the vinyl-functionalized CFs and VE, which resulted in a strong interfacial interaction and restricted the movement of resin segments.6 Possessing relative rigid phenyl group in APMA-CFs could be one of reasons for higher Tg than acryl-CFs/VE composites.
3.7. The mechanical properties of composites
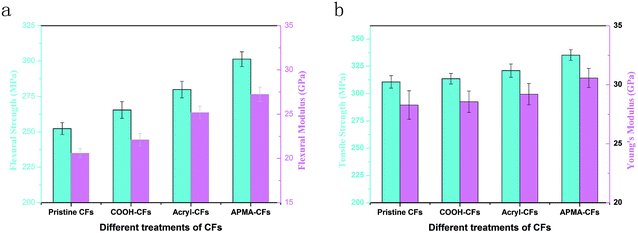
The flexural properties of the composites are influenced largely by the interfacial region between the CFs and the VE matrix.26 Fig. 10 presented the mechanical properties of the composites reinforced by pristine CFs, COOH-CFs, acryl-CFs and APMA-CFs. As shown in Fig. 10a, the flexural strength of carboxyl functionalized carbon fiber composites was increased to 265.5 MPa, 5.2% higher than pristine CFs/VE composites (252.35 MPa). It may benefit from the oxygen-containing functional groups of the carbon fibers which have the potential to create strong physical interactions like hydrogen bonding.28 The flexural strength of the acryl-CFs/VE and APMA-CFs/VE composites increased to 279.87 MPa (10.9%) and 301.3 MPa (19.4%), respectively, compared with pristine CFs/VE composites (252.35 MPa). The incremental tendency of flexural modulus was accordance with the flexural strength. The flexural modulus of the composites increases from 20.59 GPa for pristine CFs/VE composites to 27.25 GPa for APMA-CFs/VE composites. This remarkable increase in the flexural strength and flexural modulus may benefit from the covalent bonds of the vinyl functional groups on the CFs surface, which was formed during the free-radical polymerization with VE, promoting the load transfer from matrix to carbon fibers and enhancing the interfacial bonding.6,22,25The representative tensile properties of the composites are shown in Fig. 10b. The tensile strength of the pristine CFs/VE composites is 310.65 MPa which was similar with COOH-CFs/VE composites (313.54 MPa). However, the tensile strength of acryl-CFs/VE and APMA-CFs/VE composites (320.98 and 335.27 MPa) was slightly higher than pristine CFs/VE composites (310.65 MPa). This may be attributed to the promoted mechanical interlocking between the fiber and matrix resin resulting from formation of covalent bonds between the VE resins and the CFs surface. Young's modulus of the composites displays similar trend as tensile strength experiment. After treatments with acryl and APMA, the Young's modulus of the pristine CFs/VE composites (28.29 GPa) was enhanced by 3.2% and 8.1%, respectively. These slightly increments suggested effective stress transfer ability from the matrix to the CFs, probably originating from good interfacial adhesion between VE resin and carbon fiber. Note that the improvements attained in the tensile properties are lower than those achieved in the flexural strength and modulus. It could be explained that the tensile properties are mainly dominated by the fiber reinforcement, whilst the flexural properties are principally matrix-dominated.4
3.8. Interface shear strength tests
The mechanical properties of the composites depend not only on the fiber and matrix, but also on the composite's interfacial strength. In order to evaluate the interfacial strength between VE matrix and carbon fibers, the interface shear strength (IFSS) and SEM micrographs after debonding were studied as shown in Fig. 11. Micro-droplet tests were performed to investigate the enhancement effect of carbon fiber surface treatment on interfacial strength as shown in Fig. 11a. The IFSS of CF under different reaction conditions are shown in Fig. 11b. The grafting of carboxyl groups on the CF surface only leads to increase slightly in IFSS, from 33.48 MPa (CF) to 34.0 MPa (COOH-CF). However, the IFSS of acryl-CF (49.23 MPa) and APMA-CF (63.79 MPa) show an increase of 47% and 90.5% compared with the pristine CF (33.48 MPa). The improvement in IFSS may be attributed to covalent bond generated between CF and VE matrix, which results in better adhesion at the interface.19 The reinforcement effect of vinyl-CF provided a higher ability in promoting load transfer from VE resin to CF.To further reveal the role of grafted CF in interfacial enhancement, the debonded interface between various CF and VE matrix after micro-droplet test was observed as shown in Fig. 11c–f. From Fig. 11c and d, it can be clearly seen that the VE micro-droplet is entirely debonded and there is no residual matrix retaining on the CF surface. It indicates that the interface bonding is weak between the CF and VE matrix. After vinyl functionalization, there are some matrix still attach to the debonded CF surface (Fig. 11e and f). It indicates that interface between the CF and the VE matrix becomes so strong that fracturing is not restricted to the interface only. It can be ascribed to the chemical bonding between the active groups on CF and VE matrix.19,26
4. Conclusions
Vinyl-ended carbon fibers were prepared for high performance CFs/VE composites. As shown by XPS and FT-IR analysis, the grafting of vinyl groups was achieved by reaction of APMA with the carboxyl groups of carbon fiber surface. The vinyl functionalized carbon fiber and VE resin formed covalent bonds through a radical mechanism. AMPA-CFs/VE composites exhibited 19.4% and 90.5% increase of flexural strength and interface shear strength than pristine CFs/VE composites. DMA studies revealed that AMPA-CFs/VE composites exhibit considerably higher storage modulus and glass transition temperature than the pristine CFs/VE composites. However, a moderate increase in tensile properties was observed, attributed to it was mainly dominated by the fiber reinforcement. This study demonstrated the high potential of APMA-CFs as reinforcement for VE matrix based composites. The manufactured composites possess improved interface adhesion strength, suitable for a wide variety of applications such as aerospace, automotive and the energy sector.Acknowledgements
The authors acknowledge The Jilin Province Science and Technology Innovation and Achievements Transformation Project of China (20140306011GX) for financial support.References
- J. Li, Appl. Surf. Sci., 2009, 255, 8682–8684 CrossRef CAS.
- X. Wang, L. Song, W. Pornwannchai, Y. Hu and B. Kandola, Composites, Part A, 2013, 53, 88–96 CrossRef CAS.
- L. Liao, X. Wang, P. Fang, K. M. Liew and C. Pan, ACS Appl. Mater. Interfaces, 2011, 3, 534–538 CAS.
- L. Liu, L. H. Xiao, X. P. Zhang, M. Li, Y. J. Chang, L. Shang and Y. H. Ao, RSC Adv., 2015, 5, 57853–57859 RSC.
- G. Pandey, M. Wolters, E. T. Thostenson and D. Heider, Carbon, 2012, 50, 3816–3825 CrossRef CAS.
- W. H. Liao, H. W. Tien, S. T. Hsiao, S. M. Li, Y. S. Wang, Y. L. Huang, S. Y. Yang, C. C. Ma and Y. F. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 3975–3982 CAS.
- C. Deng, J. Jiang, F. Liu, L. Fang, J. Wang, D. Li and J. Wu, J. Mater. Sci., 2015, 50, 5886–5892 CrossRef CAS.
- X. Zhang, X. Fan, C. Yan, H. Li, Y. Zhu, X. Li and L. Yu, ACS Appl. Mater. Interfaces, 2012, 4, 1543–1552 CAS.
- Z. Y. Liu, B. Hao and Y. G. Zhang, RSC Adv, 2015, 5, 40668–40677 RSC.
- Y. Li, Y. Li, Y. Ding, Q. Peng, C. Wang, R. Wang, T. Sritharan, X. He and S. Du, Compos. Sci. Technol., 2013, 85, 36–42 CrossRef CAS.
- H. Rong, K.-H. Dahmen, H. Garmestani, M. Yu and K. I. Jacob, J. Mater. Sci., 2013, 48, 4834–4842 CrossRef CAS.
- S. Zhang, W. B. Liu, L. F. Hao, W. C. Jiao, F. Yang and R. G. Wang, Compos. Sci. Technol., 2013, 88, 120–125 CrossRef CAS.
- H. Cao, Y. Huang, Z. Zhang and J. Sun, Compos. Sci. Technol., 2005, 65, 1655–1662 CrossRef CAS.
- J.-Q. Li, Y.-D. Huang, S.-Y. Fu, L.-H. Yang, H.-t. Qu and G.-s. Wu, Appl. Surf. Sci., 2010, 256, 2000–2004 CrossRef CAS.
- F. Zhao and Y. Huang, Mater. Lett., 2011, 65, 3351–3353 CrossRef CAS.
- J. Yu, L. Meng, D. Fan, C. Zhang, F. Yu and Y. Huang, Composites, Part B, 2014, 60, 261–267 CrossRef CAS.
- C. Fang, J. Wang and T. Zhang, Appl. Surf. Sci., 2014, 321, 1–9 CrossRef CAS.
- H. Xu, X. Zhang, D. Liu, Y. Chun and X. Fan, Appl. Surf. Sci., 2014, 320, 43–51 CrossRef CAS.
- L. Ma, L. Meng, G. Wu, Y. Wang, M. Zhao, C. Zhang and Y. Huang, Compos. Sci. Technol., 2015, 114, 64–71 CrossRef CAS.
- F. Vautard, P. Fioux, J. Schultz, M. Nardin and B. Defoort, Appl. Surf. Sci., 2013, 286, 12–21 CrossRef CAS.
- F. Vautard, P. Fioux, L. Vidal, F. Siffer, V. Roucoules, J. Schultz, M. Nardin and B. Defoort, ACS Appl. Mater. Interfaces, 2014, 6, 1662–1674 CAS.
- Y. Wang, L. Meng, L. Fan, L. Ma, M. Qi, J. Yu and Y. Huang, Appl. Surf. Sci., 2014, 316, 366–372 CrossRef CAS.
- Y. G. Zhang, C. A. Anderson and S. C. Zimmerman, Org. Lett., 2013, 15, 3506–3509 CrossRef CAS PubMed.
- Y. G. Zhang and S. C. Zimmerman, Beilstein J. Org. Chem., 2012, 8, 486–495 CrossRef CAS PubMed.
- G. Zhang, S. Sun, D. Yang, J.-P. Dodelet and E. Sacher, Carbon, 2008, 46, 196–205 CrossRef CAS.
- C. Wang, Y. Li, L. Tong, Q. Song, K. Li, J. Li, Q. Peng, X. He, R. Wang, W. Jiao and S. Du, Carbon, 2014, 69, 239–246 CrossRef CAS.
- Q. Zehua and W. Guojian, J. Appl. Polym. Sci., 2012, 124, 403–411 CrossRef.
- F. Vautard, S. Ozcan and H. Meyer, Composites, Part A, 2012, 43, 1120–1133 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |