DOI:
10.1039/C6RA04566A
(Communication)
RSC Adv., 2016,
6, 29423-29427
Solvent-free electrospinning of UV curable polymer microfibers†
Received
20th February 2016
, Accepted 16th March 2016
First published on 17th March 2016
Abstract
The conventional solution electrospinning (e-spinning) process is facing the trouble of solvent recovery, especially for the industrial mass production of electrospun (e-spun) ultrathin fibers. This study provides a possible strategy, solvent-free e-spinning to solve this problem. By using a modified homemade e-spinning device and UV curable materials as the precursor liquid, all the spinning solution was successfully e-spun into ultrathin fibers without solvent evaporation (weight loss) in an atmosphere of nitrogen and under UV light radiation. The solidification mechanism of the fibers is ascribed to the quick curing of the acrylate bonds in the spinning stream under UV light radiation and without oxygen inhibition in the atmosphere of nitrogen. Such a break-through leads to fabrication of ultrathin fibers by solvent-free e-spinning without solution loss, and provides an eco-friendly approach to prepare new and functional (composite) ultrathin fibers by using a variety of UV curable materials and functional additions.
1 Introduction
Electrospinning (e-spinning) is an attractive and diverse approach to fabricate ultrathin polymer fibers.1–3 The electrospun (e-spun) fibers usually have diameters ranging from tens of microns to tens of nanometers and a high specific surface area, which can lead to widespread applications, such as air or liquid filtration,4 protective cloths,5 biomedical materials,6 flexible sensors,7 and battery anodes.8 A typical e-spinning device is assembled by a high-voltage power supply, a metal capillary/nozzle and a collector. e-Spinning precursor solution loaded in a syringe connecting to the metal capillary is prepared by dissolving polymers into volatile solvents with concentration usually from 5 wt% to 25 wt%. In this process, the utilization of solvents (except water) is usually unwanted especially in industrial processes due to several complications: the cost of solvents, the environmental restrictions (e.g., toxicity and inflammability), their storage, dispose and recovery. In addition, for biomedical uses, solvents must be completely removed from the e-spun fibers. To solve this limitation, solvent-free e-spinning as a promising technology has drawn much attention in recent years.
At present, solventless e-spinning mainly contains e-spinning process from melt polymers. Melt e-spinning is one of ecofriendly methods except the relatively complicated, expensive equipment and the resultant relatively coarse fibers.9–13 Despite these limitations some interesting applications have been devised, such as the melt e-spinning of a fiber mat directly onto cells12 or a hand/wound.13 Besides melt e-spinning, Levit and Tepper reported a supercritical CO2-assisted e-spinning of polydimethylsiloxane (PDMS) and poly(D,L-lactic acid) short fibers without the use of a liquid solvent.14 In this work, supercritical CO2 in fact acted as a solvent, which could reduce the polymer viscosity sufficiently to allow fibers to be e-spun from an undissolved bulk polymer sample. However, the spun fiber was only a short wire and had a remarkably large diameter (hundreds of microns). Recently, our group reported an in situ moisture curing e-spinning, namely, the precursor solution (e.g., ethyl cyanoacetate monomer, assisted by a small quantity of PMMA) thread could be cured (in situ polymerized) rapidly owing to moisture in the air to form solid ultrathin fibers and then deposits on the collector.15 However, in this work there was still part of ethyl cyanoacetate monomer (∼10 wt%) released into the air because the ethyl cyanoacetate monomer is very volatile. The follow-up studies indicate that the moisture curing e-spinning has promising application in rapid hemostasis with high efficiency and low toxicity.16,17
In this article, we propose that UV radiation may be a good way to assist solvent-free e-spinning. UV curable materials are a variety of double bond-containing monomers or oligomers, which can be completely cured under the radiation of UV light. Thanks to no volatile organic compounds (VOC) emission, they are environment-friendly and being widely used as coating, paint, ink, and adhesive, etc. However, it is well-known that an oxygen inhibition effect limits the application of UV curable materials,18 especially for high specific-surface-area fibers. In this work, by using a modified homemade e-spinning device, the spinning precursor solution (UV curable materials) can be totally e-spun into ultrathin fibers in atmosphere of nitrogen and under UV light radiation.
2 Experimental
2.1 Materials
UV curable material, DR-U301 polyurethane acrylate (PUA) was employed as precursor of e-spinning and a home-made e-spinning device in atmosphere of nitrogen was employed to fabricate ultrathin fibers. The raw materials of Etercure® DR-U301, a commercial PUA (MW ∼ 7800 and viscosity ∼ 5500 mPa s at 25 °C, Taiwan Eternal Chemical Co., Ltd, China) and photoinitiator 1173 (PI-1173, 2-hydroxy-2-methylpropio-phenone, Darocur® 1173, BASF Corporation) were commercially available and used directly without further purification.
2.2 e-Spinning apparatus
The e-spinning device (Fig. 1) for this research mainly includes a DC high-voltage power supply (HVPS, DW-P303-1ACFO, Tianjin Dongwen High Voltage, China), a metal roller collector connecting to a speed-adjustable motor installed in a transparent box, a high-pressure mercury lamp (400 W) installed 15–20 cm away from a spinneret, and N2 cylinder plugged in the box by hose. A gas probe was used to detect the concentration of oxygen in the box.
 |
| | Fig. 1 Schematic illustration of the e-spinning setup.19 | |
2.3 Preparation of solutions and e-spinning process
In a typical experiment, 8.0 g of DR-U301 and 0.4 g of photoinitiator 1173 were added into a 25 ml conical flask. The flask was packaged by aluminum foil to avoid light, and placed in 40–50 °C water, stirring with a magnetic stirrer for 1 to 2 h. Its viscosity was 5500 mPa s and 2.0 g of the resulting precursor solution was poured into a metal cone with a spinning nozzle of 1.2 cm inner diameter. The 20 cm × 20 cm × 40 cm sealed box was purged with 3 to 9 mm3 s−1 of N2 for 5 minutes, and when the concentration of oxygen decreased to less than 5%, the N2 purging speed adjusted to 0–3 mm3 s−1 to keep the oxygen concentration less than 5%. Then switch on the UV lamp, and one minute later, switch on the motor and HVPS, e-spinning began. The distance between the nozzle and the collector drum was 10–12 cm, and spinning voltage was 22 kV, the rotation speed of drum was 120 to 350 rpm. At intervals of 2 min, the e-spun fibers' weight (W1) and the weight of the rest precursor liquid (W2) were recorded by weighing the roller collector and the metal cone, respectively (Fig. 2 and Table 1). About 10 minutes later, continuous ultrathin fibers were deposited on a roller collector and taken off for characterization. For comparison, a control experiment was carried out in the air.
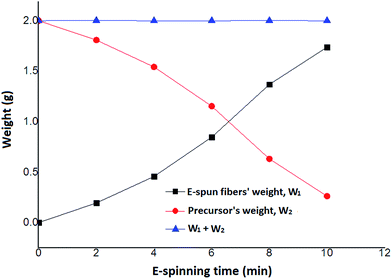 |
| | Fig. 2 Weight balance (W1 + W2) of precursor solution (W2, red circle) and the resultant fibers (W1, black square) e-spun in N2. | |
Table 1 Weight balance of precursor solution (W2) and the resultant fibers (W1) e-spun in N2
| e-Spinning time/min |
W1/g |
W2/g |
W1 + W2/g |
| 0 |
0 |
2.0000 |
2.0000 |
| 2 |
0.1945 |
1.8080 |
2.0025 |
| 4 |
0.4574 |
1.5421 |
1.9995 |
| 6 |
0.8472 |
1.1531 |
2.0003 |
| 8 |
1.3689 |
0.6315 |
2.0004 |
| 10 |
1.7382 |
0.2616 |
1.9998 |
2.4 Characterization
The e-spun fibers were characterized using an optical microscope (Olympus BX51) and a scanning electron microscope (SEM; JEOL JSM-6390). Thermal analysis was recorded by means of a thermogravimetric analyzer (TGA; Mettler-Toledo TGA/DSC). The resultant fibers e-spun in N2 and air were also compared by means of Fourier transform infrared spectroscopy (FT-IR; Nicolet AVATAR 370DTGS).
3 Results and discussion
3.1 Weight conservation and morphologies of e-spun fibers
During the e-spinning procedure in atmosphere of N2, the weight gain of the resultant fibers, W1, plus the weight of the rest precursor liquid, W2, is nearly constant and equal to the original weight of the spinning precursor, as showed in Fig. 2, indicating that the e-spinning liquid stream has cured and converted into ultrathin solid fibers completely. Namely, during the e-spinning process, there is no solvent volatilizing and no weight loss, which is quite different from conventional solution e-spinning (the solvent loss can reach 80–90 wt%). So the method reported in this work is a solvent-free and environment-friendly e-spinning procedure.
Fig. 3 shows the morphologies of DR-U301 fibers e-spun and in situ cured under UV radiation and in atmosphere of N2 and air, respectively. The resulting fibers in atmosphere of N2 are more uniform and isolated, and about 20–30 μm in diameter. For comparison, the resulting fibers e-spun in air atmosphere are sticky and conglutinated at points of contact, which demonstrates the curing is incomplete owing to the oxygen inhibition effect.
 |
| | Fig. 3 Images of the ultrathin DR-U301 fibers e-spun in atmosphere of (a) N2 and (b) air, respectively. The e-spinning voltage was 20 kV and the rotating speed of collector drum was 120 rpm. | |
3.2 Curing mechanism of e-spun fibers
In the bulk polymerization of UV materials, for example, coating film (thickness: ∼0.5 mm) of DR-U301 has good surface that is not sticky as touched. Fig. 4 exhibits the curing mechanism of the e-spun ultrathin fibers of UV curable materials under UV light radiation and in atmosphere of N2 or air. Scheme 1 demonstrates the curing process. The photoinitiator is excited to its triplet by UV light excitation, and then form radicals that initiate polymerization of monomer and oligomer containing acrylate. However, the polymerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond on the surface is in trouble. The photoinitiator triplet is quenched easily by O2 and the active radicals are scavenged to give stable peroxy ones, as shown in Scheme 2, which results in termination of polymerization.
C bond on the surface is in trouble. The photoinitiator triplet is quenched easily by O2 and the active radicals are scavenged to give stable peroxy ones, as shown in Scheme 2, which results in termination of polymerization.
 |
| | Fig. 4 Curing mechanism of the e-spun ultrathin fibers of UV curable materials under UV light radiation and in atmosphere of N2 or air. | |
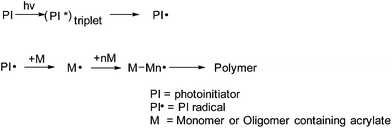 |
| | Scheme 1 Solidification process. | |
 |
| | Scheme 2 Oxygen inhibition (termination of polymerization). | |
3.3 TGA and FTIR of e-spun fibers
The thermal loss also indicates that the filaments e-spun in air were not cured thoroughly (Fig. 5a). At 200 °C, there is about 20% weight loss, which is originated from the uncured low molecular weight components. The resultant fibers e-spun in N2 and air were also compared by means of FTIR, showed in Fig. 5b. The curable double bonds, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH of polyurethane acrylate, DR-U301, have two C
CH of polyurethane acrylate, DR-U301, have two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands in FTIR spectrum located at 1636 cm−1 and 1618 cm−1 of medium intensity.20 The spectrum of filaments e-spun in N2 shows that the double bond is disappeared completely, that is to say, the curing is complete. However, the bond of C
C stretching bands in FTIR spectrum located at 1636 cm−1 and 1618 cm−1 of medium intensity.20 The spectrum of filaments e-spun in N2 shows that the double bond is disappeared completely, that is to say, the curing is complete. However, the bond of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is remained in the spectrum of filaments obtained in air, which also indicates the e-spun filaments in air have not cured completely.
C is remained in the spectrum of filaments obtained in air, which also indicates the e-spun filaments in air have not cured completely.
 |
| | Fig. 5 Characterization of DR-U301 fibers obtained by e-spinning in atmosphere of N2 and air, respectively. The spinning voltage was 20 kV and the rotating speed of collector drum was 120 rpm. (a) Thermal weight loss (TGA chart). (b) FTIR spectra. | |
3.4 Influence of rotating speed on e-spun fibers
We also investigated the influence of the collector rotating speed on the e-spun fibers. As showed in Fig. 6a–c, when the collector rotates faster and faster from 120 rpm (Fig. 3a) to 180 rpm, 210 rpm and 350 rpm, the resultant fibers arrange more orderly, and the average diameter decreases gradually than that obtained under condition of rotating speed 120 rpm (25 μm), which is 17 μm, 15 μm, and 12 μm, respectively, as shown in Fig. 6d. However, in Fig. 6c, there are some fibers conglutinated for fibers e-spun at rotating speed of 350 rpm. The possible reason is that when the rotating speed is too fast, the fibers don't have enough time to be excited enough for curing completely, so they are still sticky and then adhere to the newly e-spun fibers on the collector after 360° rotation. In addition, Fig. 6d also indicates that the distribution of average diameter of fibers becomes narrower with the increase of the collector rotating speed.
 |
| | Fig. 6 Morphologies and average diameters of the ultrathin DR-U301 fibers e-spun in atmosphere of N2. The spinning voltage was 20 kV and the rotating speed of the collector drum was (a) 180 rpm, (b) 210 rpm, and (c) 350 rpm. (d) Influence of the rotating speed on average diameter of the e-spun fibers. | |
3.5 Further discussion on UV curable e-spinning
Here, it is noted that as a UV curable material, the dose of photoinitiator 1173 could be in the range from 0.1 wt% to 10 wt%.21,22 However, a high dose of photoinitiator may result in a hard and brittle polymer coating due to low molecular weight or high crosslinking degree, while a low dose indicates a longer curing time. In this work, only an optimal dose, 4.8 wt% of photoinitiator was employed, that is to say, the weight ratio of DR-U301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PI-1173 was 20
PI-1173 was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Furthermore, this new solvent-free e-spinning method has good applicability, because UV curable materials have a variety of monomers and oligomers, and the fiber's properties can be adjusted by adding a small number of other organic or inorganic functional materials. For example, through adding different dyes or pigments into the precursor solution, color fibers could be obtained. Electrical conductive fibers could be fabricated by adding conducting polymers, carbon nanotubes, nanographene or metal nanoparticles into the precursor solution. So it is promising to fabricate some functional fibers (e.g., flame retardant, magnetic, electrical conductive, antistatic, or antibacterial fibers) through this environmentally friendly process (please see ESI, Fig. S1–S3†). At last, it should be mentioned that this approach can be applied easily to produce micro- and nano-particles as well, which are needed in several industries. And the current research progress is also helpful to 3D e-spinning (additive manufacturing or 3D printing).
1. Furthermore, this new solvent-free e-spinning method has good applicability, because UV curable materials have a variety of monomers and oligomers, and the fiber's properties can be adjusted by adding a small number of other organic or inorganic functional materials. For example, through adding different dyes or pigments into the precursor solution, color fibers could be obtained. Electrical conductive fibers could be fabricated by adding conducting polymers, carbon nanotubes, nanographene or metal nanoparticles into the precursor solution. So it is promising to fabricate some functional fibers (e.g., flame retardant, magnetic, electrical conductive, antistatic, or antibacterial fibers) through this environmentally friendly process (please see ESI, Fig. S1–S3†). At last, it should be mentioned that this approach can be applied easily to produce micro- and nano-particles as well, which are needed in several industries. And the current research progress is also helpful to 3D e-spinning (additive manufacturing or 3D printing).
4 Conclusions
In summary, a new solvent-free e-spinning technique has been proposed to successfully fabricate ultrathin fibers of UV curable materials under UV light radiation and in atmosphere of N2. In this method, all the spinning solution can be e-spun into fibers without solvent evaporation (weight loss). The as-spun fibers with cylindrical shape are uniform. For comparison, the fibers e-spun under UV light radiation and in the atmosphere of air are uneven and severe sticky. Further mechanism studies indicate that in the atmosphere of N2, the double bonds of UV curable materials can be completely cured, while in air they are remained and less cured due to strong oxygen inhibition. Namely, free radicals produced from photo initiators or monomers are scavenged easily by oxygen and become inactive. As a breakthrough of solvent-free e-spinning procedure, our method overcomes oxygen inhibition, moreover, it is eco-friendly, which provides a new route to fabricate functional (or composite) ultrathin fibers because there are many kinds of UV curable materials and functional additions widely used in industrial fields.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51373082), the Taishan Scholars Program of Shandong Province, China (ts20120528), and the Postdoctoral Scientific Research Foundation of Qingdao City.
Notes and references
- D. H. Reneker and A. L. Yarin, Polymer, 2008, 49, 2387–2425 CrossRef CAS.
- Z. M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- A. Greiner and J. H. Wendorff, Adv. Polym. Sci., 2008, 219, 107–171 CAS.
- C. Liu, P. C. Hsu, H. W. Lee, M. Ye, G. Y. Zheng, N. Liu, W. Y. Li and Y. Cui, Nat. Commun., 2015, 6, 6205 CrossRef CAS PubMed.
- X. Mao, Y. C. Chen, Y. Si, Y. Li, H. G. Wan, J. Y. Yu, G. Sun and B. Ding, RSC Adv., 2013, 3, 7562–7569 RSC.
- D. Grafahrend, K. H. Heffels, M. V. Beer, P. Grasteier, M. Möller, G. Boehm, P. D. Dalton and J. Groll, Nat. Mater., 2011, 10, 67–73 CrossRef CAS PubMed.
- X. Liu, J. Y. Wu, L. L. Gu, Q. P. Zhang, J. Y. Wu, Y. Z. Long and Z. Y. Fan, Nat. Commun., 2014, 5, 4007 CAS.
- H. Wu, G. Chan, J. W. Choi, I. Ryu, Y. Yao, M. T. McDowell, S. W. Lee, A. Jackson, Y. Yang, L. B. Hu and Y. Cui, Nat. Nanotechnol., 2012, 7, 310–315 CrossRef CAS PubMed.
- J. Lyons, C. Li and F. Ko, Polymer, 2004, 45, 7597 CrossRef CAS.
- E. Zhmayev, D. Cho and Y. L. Joo, Polymer, 2010, 51, 4140–4144 CrossRef CAS.
- R. J. Deng, Y. Liu, Y. Ding, P. C. Xie, L. Luo and W. M. Yang, J. Appl. Polym. Sci., 2009, 114, 166–175 CrossRef CAS.
- P. D. Dalton, K. Klinkhammer, J. Salber, D. Klee and M. Möller, Biomacromolecules, 2006, 7, 686–690 CrossRef CAS PubMed.
- C. C. Qin, X. P. Duan, L. Wang, L. H. Zhang, M. Yu, R. H. Dong, X. Yan, H. W. He and Y. Z. Long, Nanoscale, 2015, 7, 16611–16615 RSC.
- N. Levit and G. Tepper, J. Supercrit. Fluids, 2004, 31, 329–333 CrossRef CAS.
- S. L. Liu, Y. Z. Long, Y. Y. Huang, H. D. Zhang, H. W. He, B. Sun, Y. Q. Sui and L. H. Xia, Polym. Chem., 2013, 4, 5696–5700 RSC.
- R. H. Dong, C. C. Qin, X. Qiu, X. Yan, M. Yu, L. Cui, Y. Zhou, H. D. Zhang, X. Y. Jiang and Y. Z. Long, Nanoscale, 2015, 7, 19468–19475 RSC.
- K. Jiang, Y. Z. Long, Z. J. Chen, S. L. Liu, Y. Y. Huang, X. Y. Jiang and Z. Q. Huang, Nanoscale, 2014, 6, 7792–7798 RSC.
- K. Studer, C. Decker, E. Beck and R. Schwalm, Prog. Org. Coat., 2003, 48, 92–100 CrossRef CAS.
- Y. Z. Long, H. W. He, L. Wang, L. H. Zhang, X. P. Duan, R. H. Duan, C. C. Qin, H. Zhao and L. H. Xia, China Patent 2015105267649 and 2015206431345, 2015.
- O. Belaidi, T. Bouchaour and U. Maschke, Org. Chem. Int., 2013, 14, 348379 Search PubMed.
- G. Li, S. Jiang, Y. Gao, X. Liu and F. Sun, Ind. Eng. Chem. Res., 2013, 52, 2220–2227 CrossRef CAS.
- H. Lee, S. G. Lee and P. S. Doyle, Lab Chip, 2015, 15, 3047–3055 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04566a |
| ‡ These authors contributed to this work equally. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond on the surface is in trouble. The photoinitiator triplet is quenched easily by O2 and the active radicals are scavenged to give stable peroxy ones, as shown in Scheme 2, which results in termination of polymerization.
C bond on the surface is in trouble. The photoinitiator triplet is quenched easily by O2 and the active radicals are scavenged to give stable peroxy ones, as shown in Scheme 2, which results in termination of polymerization.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH of polyurethane acrylate, DR-U301, have two C
CH of polyurethane acrylate, DR-U301, have two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching bands in FTIR spectrum located at 1636 cm−1 and 1618 cm−1 of medium intensity.20 The spectrum of filaments e-spun in N2 shows that the double bond is disappeared completely, that is to say, the curing is complete. However, the bond of C
C stretching bands in FTIR spectrum located at 1636 cm−1 and 1618 cm−1 of medium intensity.20 The spectrum of filaments e-spun in N2 shows that the double bond is disappeared completely, that is to say, the curing is complete. However, the bond of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is remained in the spectrum of filaments obtained in air, which also indicates the e-spun filaments in air have not cured completely.
C is remained in the spectrum of filaments obtained in air, which also indicates the e-spun filaments in air have not cured completely.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PI-1173 was 20
PI-1173 was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Furthermore, this new solvent-free e-spinning method has good applicability, because UV curable materials have a variety of monomers and oligomers, and the fiber's properties can be adjusted by adding a small number of other organic or inorganic functional materials. For example, through adding different dyes or pigments into the precursor solution, color fibers could be obtained. Electrical conductive fibers could be fabricated by adding conducting polymers, carbon nanotubes, nanographene or metal nanoparticles into the precursor solution. So it is promising to fabricate some functional fibers (e.g., flame retardant, magnetic, electrical conductive, antistatic, or antibacterial fibers) through this environmentally friendly process (please see ESI, Fig. S1–S3†). At last, it should be mentioned that this approach can be applied easily to produce micro- and nano-particles as well, which are needed in several industries. And the current research progress is also helpful to 3D e-spinning (additive manufacturing or 3D printing).
1. Furthermore, this new solvent-free e-spinning method has good applicability, because UV curable materials have a variety of monomers and oligomers, and the fiber's properties can be adjusted by adding a small number of other organic or inorganic functional materials. For example, through adding different dyes or pigments into the precursor solution, color fibers could be obtained. Electrical conductive fibers could be fabricated by adding conducting polymers, carbon nanotubes, nanographene or metal nanoparticles into the precursor solution. So it is promising to fabricate some functional fibers (e.g., flame retardant, magnetic, electrical conductive, antistatic, or antibacterial fibers) through this environmentally friendly process (please see ESI, Fig. S1–S3†). At last, it should be mentioned that this approach can be applied easily to produce micro- and nano-particles as well, which are needed in several industries. And the current research progress is also helpful to 3D e-spinning (additive manufacturing or 3D printing).




