Effect of the mass ratio of micron and submicron silver powder in the front electrode paste on the electrical performance of crystalline silicon solar cells
Hanying Wangac,
Yuping Taib,
Ruixiao Lib,
Hui Wang*ab and
Jintao Bai*a
aNational Key Laboratory of Photoelectric Technology and Functional Materials (Culture Base), National Photoelectric Technology and Functional Materials, Application of International Science and Technology Cooperation Base, Institute of Photonics and Photon-Technology, Northwest University, Xi'an 710069, People's Republic of China. E-mail: huiwang@nwu.edu.cn; jintaobai@sina.cn; baijt@nwu.edu.cn; Fax: +86 29 8830 3798; Tel: +86 29 8836 3115 Tel: +86 29 8830 3877
bKey Laboratory of Synthetic and Natural Functional Molecule Chemistry (Ministry of Education), College of Chemistry and Materials Science, Northwest University, Xi'an 710069, People's Republic of China
cCollege of Energy Engineering, Yulin University, Yulin, 719000, People's Republic of China
First published on 10th March 2016
Abstract
In this paper, highly-dispersed spherical micron-sized (D50 = 2.94 μm) and submicron-sized (D50 = 0.59 μm) silver powders were prepared by a chemical reduction method. Hybrid silver powder was then synthesized by mixing the micron- and submicron-sized silver powders, and the mass percentages of submicron-sized silver powder in the hybrids were 0, 100, 5, 10, 15, and 20%. The tap density of the silver powders, cross-sectional and surface microstructures, and performance of cells designed using these silver powders were investigated. The results suggested that the mass ratio of hybrid silver powder had an important influence on the structure of the contact interface, the quality of the ohmic contacts, and the electrical performance of solar cells. The electrical performances of solar cells made with mixed silver powder were better than those made using pure micron- or submicron-sized silver powders. The optimal level of submicron-sized silver powder content was 15 wt%. This was because the surface morphology of the thick films prepared using this hybrid was smooth and dense, the layer had sufficient silver crystallites to increase the contact area fraction, and there was a thin glass layer to improve the probability of tunnelling from Ag crystallites to the Ag grid. This led to the formation of good ohmic contacts and conductive chains. As a result, this silver powder gave the best photoelectric conversion efficiency (18.282%) and series resistance (0.0019 Ω).
Introduction
With increasing concerns about environmental pollution, global climate change, and the high cost of nuclear power, solar energy has attracted significant attention in recent years as a clean, safe, effective, abundant, and renewable alternative energy source.1–5 Crystalline silicon (c-Si) solar cells are the most widely used photovoltaic (PV) cells and have around a 90% market share in total global PV cell production.6 Front-contact silver paste is a key material in high-efficiency c-Si solar cells because it is an essential channel for photocurrent conduction. Additionally, silver paste has become one of the major costs of solar cell production due to the rapid reduction in the price of silicon. Therefore, extensive research has been carried out to improve the performance of silver paste.7–16Currently, silver conductive thick films formed by screen-printing are widely used as metallization contacts in c-Si solar cells. The silver thick film paste primarily contains silver powder (as a conductive phase for electron collection), glass frits (as a binder phase to stick the silver powders to the silicon substrate and form good ohmic contacts), and an organic medium (as a disperse phase to allow smooth printing of the paste and obtain a good aspect ratio of grids).17 Silver powders are the major components (70–90 wt%) of the paste, because silver has an excellent electrical conductivity.18–20 As the efficiency of electrical conduction is largely dependent on the connection of filler particles and good-quality thick-film ohmic contacts. Optimizing the silver particle size of the silver paste can help achieve continuous linkage of filler particles and good-quality thick-film ohmic contacts. However, it is not true that particles can contact well everywhere between the micro-sized particles.21–23 Although contact between metallic particles seems necessary to achieve electrical conductivity, it has been observed that some conductivity can be obtained in systems where the particles are not necessary in contact; a carrier tunneling mechanism was proposed to explain this phenomenon. However, for tunneling to work, inter-particle distances of less than 10 nm are required,24,25 and hence when micron-sized silver particles are not sufficiently connected, the addition of a small amount of submicron-sized silver particles helps to build a conductive network and achieve good-quality thick-film ohmic contacts.
In this study, highly dispersed spherical micron- and submicron-sized silver powders were prepared by a chemical reduction method. The silver front contact pastes were prepared by mixing micron- and submicron-sized silver powders in various ratios. The silver conductive thick films were formed by screen-printing. The photovoltaic performances of c-Si solar cells utilizing the silver paste were investigated. The effects of silver particle size were studied and exploited to improve silver paste composition for high sheet resistance emitters using rapid firing conditions.
Experimental
Preparation of micron- and submicron-sized silver powders
Micron- and submicron-sized silver powders were prepared by a chemical reduction method using silver nitrate as the source of silver, gum arabic as a dispersant, and ascorbic acid as a reducing agent. Silver nitrate (1.2 mol L−1) was reduced by ascorbic acid (0.8 mol L−1) in the presence of gum arabic (gum arabic/silver nitrate (w/w) = 0.025).25,26 The pH of the mixture solution was controlled by adding a certain volume of ammonium hydroxide. The reaction continued for 0.5 h using a variable high-speed stirrer (4500 rpm) until silver nitrate was completely reduced. The whole procedure was carried out at room temperature. The synthesized silver powders were washed with distilled water and ethanol for three times and subsequently centrifuged and dried at 60 °C for 5 h. The average particle sizes and size distributions were determined using a laser size distribution analyzer (BT-9300-H, Dandong City Baxter Instrument Co., Ltd.). The tap densities of the powders were measured using a particle density tester (Hylology, HY-100). The electrical resistivity measurements were carried out using a four-point probe metal/semiconductor resistivity meter (Shanghai Peak Electronic Instrument Co., Ltd., SB100A/20A, China). The particle sizes, tap densities, and electrical resistivities of the spherical silver powders are shown in Table 1. The particle size distributions of spherical silver powders are shown in Fig. 1.| No. | Sizes (μm) | TD (g cm−3) | Resistivity (Ω m) | ||
|---|---|---|---|---|---|
| D10 | D50 | D90 | |||
| SA | 1.94 | 2.94 | 4.05 | 6.25 | Infinite |
| SB | 0.38 | 0.59 | 0.86 | 4.49 | 1.164 × 10−4 |
 | ||
| Fig. 1 Particle size distributions of spherical silver powders with different sizes: (a) (D50 = 2.94 μm), (b) (D50 = 0.59 μm). | ||
Mixing of micro-size and submicro-size silver powders
The micron-sized silver powders (SA) and submicron-sized silver powders (SB) were poured into a beaker filled with alcohol in various weight ratios and mixed by hand stirring, followed by 20 min of ultrasonic processing. Small amounts of submicron-sized silver powders were added to micron-sized silver powders. The compositions, tap densities, and electrical resistivities of the hybrids are shown in Table 2.| Silver powder | Micro-Ag (wt%) | Submicro-Ag (wt%) | TD (g cm−3) | Resistivity (Ω m) | Paste | Viscosity (pa s) | Thixotropy | Cell | Aspect ratio | Printing quality |
|---|---|---|---|---|---|---|---|---|---|---|
| SA | 100% | — | 6.125 | Infinite | PA | 662 | 8.67 | CA | 0.218 | Good |
| SB | — | 100% | 4.491 | 1.6 × 10−6 | PB | 258 | 6.03 | CB | 0.162 | Very bad |
| SH1 | 95% | 5% | 6.168 | Infinite | PH1 | 434 | 6.61 | CH1 | 0.295 | General |
| SH2 | 90% | 10% | 6.355 | 5.351 | PH2 | 397 | 6.76 | CH2 | 0.310 | Good |
| SH3 | 85% | 15% | 6.673 | 3.311 × 10−4 | PH3 | 412 | 7.32 | CH3 | 0.382 | Very good |
| SH4 | 80% | 20% | 6.277 | 2.708 × 10−4 | PH4 | 476 | 6.83 | CH4 | 0.302 | General |
Preparation of silver thick film
The silver pastes (denoted as PA, PB, PH1, PH2, PH3, and PH4) were prepared by mixing pre-mixed silver powder, Pb-based glass frit powders, and an organic vehicle (terpineol and ethyl cellulose) in the ratio 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (wt%), using a three-roller machine (Puhler, PTR65C). The viscosities and thixotropic index of these pastes were measured using a viscometer (Brookfield, DV-II+ PRO, USA), the results are shown in Table 2. Then, the silver pastes were screen-printed on the front side of p-type polycrystalline silicon wafers (sheet resistance: 60 Ω sq−1, size: 156 mm × 156 mm). Each type of silver paste was printed on at least three wafers every time and more than once. Subsequently, the wafers were dried in an oven in air for 30 min at 60 °C.
12 (wt%), using a three-roller machine (Puhler, PTR65C). The viscosities and thixotropic index of these pastes were measured using a viscometer (Brookfield, DV-II+ PRO, USA), the results are shown in Table 2. Then, the silver pastes were screen-printed on the front side of p-type polycrystalline silicon wafers (sheet resistance: 60 Ω sq−1, size: 156 mm × 156 mm). Each type of silver paste was printed on at least three wafers every time and more than once. Subsequently, the wafers were dried in an oven in air for 30 min at 60 °C.
Measurements
The microstructures of the silver powders were investigated by X-ray diffraction (XRD) using a diffractometer (D/Max-3C) with Cu-Kα radiation (k = 0.54184 nm) at a tube voltage of 45 kV and a tube current of 200 mA. The morphologies of the silver powders, silver pastes, and silver electrode samples were studied using a scanning electron microscope (SEM, Quanta 400 FEG instrument, Oxford INCA 35 detector, 25 kV), as were cross-sections of the solar cells.26–28 The PV characteristics of the c-Si solar cells were measured using an EKO I–V tracer under standard testing conditions (STC): solar radiation was 1000 W m−2 and temperature was 25 °C.The surface morphology of the cells was obtained by chemical etching, which consisted of two steps: (a) etching by aqua regia (32% HCl, 65% HNO3, v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; t = 1 h) to remove silver from the finger bulk; (b) subsequent etching of the glass layer by a 3% HF solution for 10 min at room temperature, to remove the glass layer at the emitter surface.
1; t = 1 h) to remove silver from the finger bulk; (b) subsequent etching of the glass layer by a 3% HF solution for 10 min at room temperature, to remove the glass layer at the emitter surface.
Results and discussion
Microstructure and morphology of spherical silver powders
Spherical silver powders of different sizes were prepared in a typical manner. The sizes of the prepared silver particles gradually became larger as the concentration of ascorbic acid increased, when silver nitrate concentration and mass ratio of gum arabic to silver nitrate were kept constant. This was because the high reactant concentration resulted in a high reaction rate that improved probability of collisions between primary silver powders in the system. However, an opposite trend in the size of silver particles was observed when the dosage of dispersant was increased, i.e., as the mass ratio of gum arabic to silver nitrate increased, the size of silver particles decreased and the size distribution narrowed, as shown in Table 1. Fig. 2 shows the XRD patterns of the silver particles synthesized from silver nitrate solutions. It can be seen that all the peaks in the XRD patterns are in accordance with standard crystalline silver (JCPDS file no. 04-0783), implying that these silver powders were of high purity. The high intensity of the peaks also indicates that the silver powders had good crystallinity. Fig. 3 shows the SEM surface morphologies of the silver powders, which indicate that the silver powders were highly dispersed with good size distributions. Highly dispersed silver powders with high tap densities are required for the silver paste, to ensure fabrication of an even, dense, and conductive thick film after the sintering process.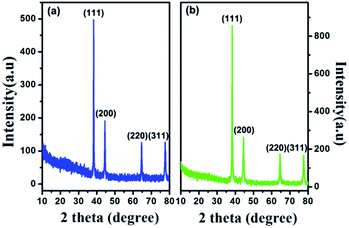 | ||
| Fig. 2 XRD patterns of spherical silver powders with different sizes: (a) (D50 = 2.94 μm), (b) (D50 = 0.59 μm). | ||
 | ||
| Fig. 3 SEM images of spherical silver powders with different sizes: (a) (D50 = 2.94 μm), (b) (D50 = 0.59 μm). | ||
Effect of hybrid silver powder tap density on the front electrode grid line
Due to the increased spaces between particles, the powder is usually less dense than the corresponding pastes. Granular stacking can hinder the densification of grid lines, affecting the electrical performance of the solar cell. In order to improve the performance of the solar cell, the density of the silver powder was measured, and then the quantity of organic vehicle aligned with this as closely as possible in order to make the particles pile up. In this paper, four hybrids of silver powders were prepared by mixing different mass ratios of SA and SB. The compositions (wt%) are shown in Table 2. Tap density is one of the characteristic parameters of a powder, which represents the degree of densification of the powder. As we know, the degree of densification increases with increasing tap density. Fig. 4 shows tap densities of the hybrid powders and the SEM morphologies of the hybrid powders are shown in Fig. 5. Voids and gaps noticeably decreased as the submicron-sized silver powder content increased. However, as more submicron-particles were added, more aggregates appeared, and more spaces between aggregates and microparticles formed.The SEM morphologies of the silver pastes are shown in Fig. 6, indicating the distribution of micron- and submicron-sized silver powders, and glass frits in the organic vehicle. The SEM images show the materials were mixed homogeneously in the vehicle. The gaps between the micron-sized silver powders first decreased and then increased as the mass fraction of submicron-sized silver powders increased.
 | ||
| Fig. 6 SEM morphologies of the silver pastes with different mass ratio: (a) PA, (b) PB, (c) PH1, (d) PH2, (e) PH3, (f) PH4. | ||
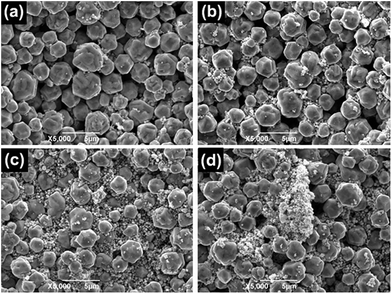
Solar cells were fabricated with the silver pastes by silkscreen printing and drying. The solar cells were fired at peak temperatures of 900 °C. The surface morphologies of the thick silver films of six solar cells are shown in Fig. 7. There are a few big holes on the surface of the solar cell made with paste PB (Fig. 7b). There are many small holes on the surface of the solar cells made with pastes PA, PH1, PH2, and PH4, and these surfaces are also not smooth or flat (Fig. 7a, c, d and f). However, there are barely any holes on the surface of the solar cell made with paste PH3, and this surface is relatively smooth and flat (Fig. 7e). There is a more dense thick film surface for the solar cell made with paste PH3, suggesting that the tap density of the silver powder has important effect on thick film microstructure.
 | ||
| Fig. 7 The surface morphology of six silver thick films after firing: (a) CA, (b) CB, (c) CH1, (d) CH2, (e) CH3, (f) CH4. | ||
Fig. 8 shows cross-sections of the silver electrodes. Greater tap density aided the formation of a more compact microstructure and conductive network; this resulted in formation of good current conductions, which improved the photoelectric conversion efficiency of the solar cells. This shows that mixing submicron- and micron-sized powders is a good way to improve solar cell performance.
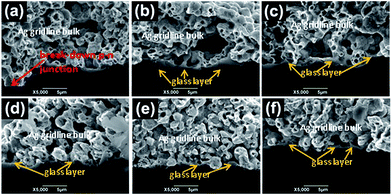 | ||
| Fig. 8 Cross-sectional SEM micrographs of the front electrode grid line after firing: (a) CA, (b) CB, (c) CH1, (d) CH2, (e) CH3, (f) CH4. | ||
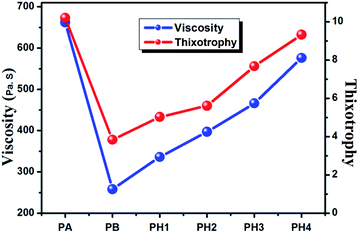
Effect of viscosity and thixotropic index of pastes on the front electrode grid line
The viscosity and thixotropic index of the pastes have important influences on printing quality and film thickness. Fig. 9 shows the viscosities and thixotropic index of the pastes. These pastes were screen printed on Si3N4 polycrystalline silicon solar cells with printed aluminum rear electrodes and silver back field, and were dried. Pictures of the grid lines of the solar cells are shown in Fig. 10. It can be seen in Fig. 10a that the grid line prepared using PA broke because the silver paste was too dense and its viscosity index was too large, and Fig. 10b shows that the grid line prepared using PB had serious spill points and broke because the silver paste was watery and its viscosity index was too little. Fig. 10c, d and f illustrate that the grid lines prepared using PH1, PH2, and PH4 had slight spill points. However, the grid line prepared using PH3 showed a perfectly smooth pattern (Fig. 10e). This indicates that when the viscosity index of the silver paste was too large and the paste was also too dense, which was a disadvantage when printing, it resulted in break points and broken grids. This prevented effective formation of ohmic contacts and hindered current transmission, thus affecting the electrical performance of the solar cells. On the other hand, when viscosity index of paste is less, the paste was watery, and the front electrode grid line was wide and irregular, with spill points and broken grids, which decreased the area capable of receiving light, reducing solar cell performance. Furthermore, the desirable ohmic contact and current transmission can't be effectively formed, affecting the electrical performance of solar cells. This indicates that the viscosity of silver pastes has an important influence on their screen-printability. | ||
| Fig. 10 SEM picture of the front electrode grid line with six kinds of different silver pastes: (a) CA, (b) CB, (c) CH1, (d) CH2, (e) CH3, (f) CH4. | ||
The thixotropic index of silver pastes has a significant influence on the height and width of the solar cell grid line. With increasing thixotropic index, the height of the grid line increased and its width decreased; thus, when thixotropic index is larger, the printing properties of the paste will be very poor. In the end, the front electrode grid line will appear irregular, and spill points and broken grids will occur. In addition, when thixotropic index is smaller, the height of the grid line is lower and its width increases, resulting in increased shading losses and reduced solar cell efficiency. In fact, the electrodes on the front surface of a c-Si solar cell must be as fine as possible, while also being thick enough to minimize power losses.
The cross-sectional SEM image in Fig. 11 shows the aspect ratio of the thick film electrodes for six front electrode grid lines after sintering, which were 0.334, 0.205, 0.306, 0.366, 0.503, and 0.338. The aspect ratio of the grid line for solar cell CH3 was the largest, while solar cell CB had the smallest aspect ratio.
 | ||
| Fig. 11 Cross-sectional SEM micrographs of the front electrode grid line after sintering: (a) CA, (b) CB, (c) CH1, (d) CH2, (e) CH3, (f) CH4. | ||
Effect of submicron mass percentage on contact interface structure
Cross-sectional SEM was performed at the contact interface to explain the ohmic contact. Fig. 12 shows the SEM images of the Ag–Si contact interface formed at peak temperatures of 900 °C using pastes with six different silver powder mass ratios and the schematic of the solar cells is shown in Fig. 13. Fig. 12a shows that the larger micron-sized silver powders gave rise to large silver crystallites with a widely varying thickness of the glass layer between the silver gridline and the silicon emitter, which was ultra-thin in some regions and thick in others. There was also direct Ag bulk/Ag crystallite connections observed in this paste. Fig. 12b shows that the use of submicron-sized silver powders gave rise to a fairly uniform and thick glass layer between the silver gridline and the silicon surface in most regions. In addition, spherical silver particles were found to be suspended in the glass layer. The presence of a thick glass layer resulted in high contact resistance, high series resistance (Rs), and lower open-circuit voltage (Voc) and fill factor (FF). Fig. 12c–f show that hybrid silver powders gave many small silver crystallites embedded into the silicon emitter surface along with a thin glass layer in many regions. As submicron silver powder content increased, the number of silver crystallites first increased and then decreased. A large number of silver crystallites gave higher contact area fraction, and a thin glass layer improved the probability of tunneling from silver crystallites to the silver grid. This may explain why CH3 had lowest Rs (0.0019 Ω) and highest efficiency (Eff, 18.282%). | ||
| Fig. 13 The schematic of the solar cells: (a) cell with micron-sized silver powders, (b) cell with submicron-sized silver powders, (c) cell with hybrid silver powders. | ||
Fig. 13a shows large micron-sized silver powders which slows the sintering process, resulting in more dissolution of silver into the glass, which in turn leads to a large number of larger silver precipitates or crystallites at the interface,20 and which can breakdown p–n junction. Fig. 12b shows submicron-sized silver powders causing sinter rapidly during sintering, which may form thick glass layer between the silver gridline and the silicon surface and reduce the dissolution of Ag into the glass frit. Fig. 12c shows hybrid silver powders which can create more small silver crystallites in thin glass layer.
It has been suggested that silver crystallites serve as current pickup points and that conduction from the silver crystallites to the bulk of the silver grid takes place via tunneling through the ultrathin glass layer above some of these crystallites.20 As a result, the size, number, and distribution of these silver crystallites as well as the thickness of the glass layer play a critical role in determining contact, series, and shunt resistances of the cell. For example, ultrafine silver crystallites would be suspended in the thick glass layer and increase series resistance (Rs) of solar cell. But small Ag crystallites would be easily for the tunnelling through the thin glass layer. Similarly, if the size of the crystallites become too large, which can increase the tunneling probability and current transport, but it would start to approach or exceed the p–n junction depth.
Effect of submicron mass percentage on Ag crystallites grown on Si substrate
In order to investigate the influence of the mass percentage of submicron silver powder on the silver crystallite distribution in the glass layer and on the silicon substrate, the different hybrid silver powders (SA, SB, SH1, SH2, SH3, and SH4) were mixed with glass frits and an organic medium to prepare front-side silver pastes and printed onto silicon substrates to form positive emitters, denoted CA, CB, CH1, CH2, CH3, and CH4. In Fig. 14, the silver bulks were removed by a chemical etching technique using aqua regia to expose the silver crystallites in the glass layer and the depth of the glass layer.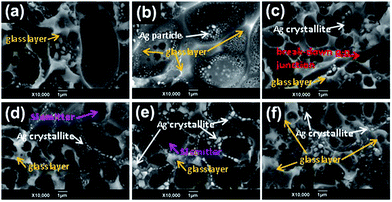 | ||
| Fig. 14 SEM top-view images of the c-Si solar cell after the first chemical etching using aqua regia: (a) CA, (b) CB, (c) CH1, (d) CH2, (e) CH3, (f) CH4. | ||
During the sintering process, the lead silicate glass softened and melted, and then started to dissolve the silver particles. Upon further heating, this mixture of glass frits and silver fluidized and started to etch the silicon nitride layer via the redox reaction below:20,26–28
| 2PbO + SiNx → 2Pb + SiO2 + N2 |
| PbO + Si → Pb + SiO2 |
Finally, upon cooling, the silver and lead separated according to the phase diagram, with silver crystallizing at the silicon surface.
It can be clearly seen in Fig. 14a that much larger silver crystallites were distributed in the glass layer and embedded in the silicon substrate, which would break down the p–n junction and lead to electric leakage from the solar cell. According to the sintering model, larger particles should lead to even more silver dissolution in the glass frit, and consequently, at a few locations the bulk of the silver gridline was observed to be in direct contact with the silicon emitter. This makes the device more vulnerable to shunting for high sheet resistance shallow emitters. Fig. 14b shows that submicron-sized silver powders might not sinter during the fast firing process, remaining trapped or suspended in the glass layer. In this case, the precipitate did not penetrate the glass layer, so an effective ohmic contact could not be acquired. In cells with hybrid silver powders, a few small silver crystallites were distributed uniformly in the glass layer, as displayed in Fig. 14c–f, and the number of silver crystallites first increased and then decreased with increasing content of submicron particles in the mixed silver powder. The glass was thin in many regions and silver crystallites were distributed quite uniformly and were observed across most of the Ag–Si interface, offering conductive channels and mild etching of the silicon substrate (Fig. 14e), meeting the requirements for good ohmic contact.
Silver concentration at the surface of the Si emitter was measured by Energy Dispersive Spectrometer (EDS). To observe the silver crystallites at the surface of the contact interface, we first etched the silver grid using aqua regia, followed by etching the glass layer with HF, without affecting the silver crystallites embedded into the silicon emitter. Fig. 15 shows the surface SEM and EDS of the contact interface for cells with different mixed silver powders. In the case of micron-sized silver powder, sintering took longer because surface interdiffusion was not as rapid as with submicron-sized silver powder. This provided more time, surface area, and opportunity for silver dissolution in the glass frit before sintering. As a result, solid blocks of silver were formed at the interface, mostly in direct contact with the silicon emitter, and these were etched with aqua regia leaving a large number of pits, as shown in Fig. 15a. In the case of submicron-sized silver powder, silver sintering and surface interdiffusion between particles both occurred rapidly, and a lot of silver could dissolve in the glass frit during firing. Less silver crystallites appeared on the silicon emitter surface during cooling (Fig. 15b). Fig. 15c–f show that the silver concentration first increased and then decreased with increasing submicron silver powder content in the mixed silver powder. The maximum silver mass fraction on the silicon emitter surface of the cell was for CH3 (Fig. 15e), and the series resistance of this cell was the lowest. Note the silver concentration on the silicon emitter surface had an important effect on the electrical performance of the cells.
 | ||
| Fig. 15 SEM and EDS images of Ag concentration at the p–n junction after the second chemical etching using HF acid: (a) CA, (b) CB, (c) CH1, (d) CH2, (e) CH3, (f) CH4. | ||
Electrical performance of cells fabricated with different silver pastes
The electrical performance of the cells fabricated with different silver pastes was investigated, and the results are shown in Table 3. The cells fabricated with mixed silver powder had smaller series resistances and higher photoelectric conversion efficiencies than those fabricated with pure micron or submicron silver powders. The photoelectric conversion efficiency of the cells was different for different mass ratios of micron and submicron silver powder, the maximum efficiency (18.282%) of cell CH3 was 0.629 percentage points higher than the efficiency of cell CH1 (17.653%). From Table 3 we can find that the ratio of silver powders had an important effect on the electrical performance of solar cells. The major reason may be as follows: large micron-sized silver powders slow the sintering process and result in the formation of solid blocks of silver, which would breakdown p–n junction, then junction leakage current density (Jo2) would increase and shunt resistance (Rsh), Voc, and FF of the cell would decrease. The submicron-sized silver powders, the easier the interdiffusion of atoms, the quicker the sintering, resulting in the formation of thick glass layer and smaller dissolution of silver into the molten glass layer. At last Rs would be less and Voc, FF, and Eff would be higher. Similarly Rs first decreased and then increased and Voc, Isc, Rsh, FF, and Eff of the cells first increased and then decreased with increasing submicron silver powder content in the hybrids. Based on the above results and observations we found that the mass ratio of hybrid silver powder had an important influence on the structure of the contact interface, the quality of ohmic contacts, and the electrical performance of solar cells.| Silver powders | Paste | Cell | Voc (V) | Isc (A) | Vmp (V) | Imp (A) | Pmp (W) | Rs (Ω) | Rsh (Ω) | FF (%) | Eff (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | PA | CA | 0.631 | 8.628 | 0.519 | 7.972 | 4.138 | 0.0039 | 14.155 | 76.002 | 17.006 |
| SB | PB | CB | 0.626 | 7.921 | 0.460 | 5.694 | 2.622 | 0.0232 | 24.020 | 52.894 | 10.773 |
| SH1 | PH1 | CH1 | 0.635 | 8.771 | 0.525 | 8.183 | 4.296 | 0.0036 | 35.133 | 77.086 | 17.653 |
| SH2 | PH2 | CH2 | 0.630 | 8.952 | 0.523 | 8.399 | 4.394 | 0.0020 | 38.430 | 77.813 | 18.056 |
| SH3 | PH3 | CH3 | 0.635 | 8.945 | 0.530 | 8.394 | 4.449 | 0.0019 | 38.895 | 78.385 | 18.282 |
| SH4 | PH4 | CH4 | 0.635 | 8.705 | 0.532 | 8.153 | 4.334 | 0.0021 | 37.043 | 78.313 | 17.811 |
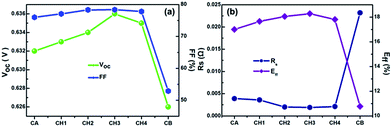
With increasing submicron silver powder content, the Voc, FF, and Eff of the cells first increased and then decreased, with the maximum occurring for a submicron silver powder content of 15 wt%, as shown in Fig. 16. This shows that there is an optimal level of submicron silver powder content in mixed silver powders. The reason for this may be that as the content of submicron silver powder increases, more and more clusters of submicron particles “float” among bigger particles. Some of them act as bridges to connect big ones, which can help them conduct. However, when the content of submicron silver powder continues to increase, some submicron particles form isolated islands, keeping their distance from other particles or clusters. This reduces the possibility of continuous linkages forming. Thus, the chances of direct contact between micron-sized particles are less with introduction of a large number of submicron particles, meaning the efficiency of solar cells eventually begins to decline.
In addition, with increasing submicron content, the series resistance of the cells first decreased and then increased, attaining a minimum value with a submicron silver powder content of 15 wt% (Fig. 16). A possible reason could be as follows: for a solar cell using silver powder, the bulk resistivity consists of three main contributions: the bulk resistance of the silver powder, the contact resistance, and the tunneling resistance between two particles in the conductive network. When pure micron silver powder is used as conductive filler, although the bulk resistance is very low, for the same filler content, the number of particles is less because of their larger size. Thus, the probability of particle contact decreases, which might result in a less conductive network in the paste; consequently, the tunneling resistance would be increased. At the same time, larger silver particles gave rise to large silver crystallites with a widely varying thickness of the glass layer between the silver gridline and the Si emitter, which might result in high contact resistance. As a result, series resistance would be increased. On the other hand, when a small amount of submicron silver powder is added to the micron silver powder, the probability of two particles contacting is higher, which might result in lowering the series resistance of the solar cell. However, with further increase in the content of submicron silver powder, the number of contact points increases, which enhances the contact resistance and eventually the series resistance of the solar cell increases. This observation reveals that the mass ratio of mixed silver powder has a drastic effect on the series resistance of solar cells.
Conclusions
Positive progress in the development of silver metallization has recently led to significant efficiency improvements for standard crystalline silicon solar cells. In this study, we prepared highly dispersed spherical micron-sized (D50 = 2.94 μm) and submicron-sized (D50 = 0.59 μm) silver powders. Hybrid silver powder was then synthesized by mixing micron- and submicron-sized silver powders. The mass percentages of submicron-sized silver powder in the hybrid powders were 0, 100, 5, 10, 15, and 20%. The mass ratio of hybrid silver powder had an important influence on the structure of the contact interface, the quality of ohmic contacts, and the electrical performance of solar cells. A number of comparative experiments showed that the electrical performance of solar cells fabricated with mixed silver powder was better than those using pure micron- or submicron-sized silver powder. Meanwhile, the optimal content of submicron silver powder was 15 wt%, that is to say, the optimum mixed silver powder was SH3. It was found that this hybrid silver powder gave the best photoelectric conversion efficiency (18.282%) and series resistance (0.0019 Ω). That was because the surface morphology of the thick films prepared using SH3 silver powder was smooth and dense, and had sufficient silver crystallites to increase the contact area fraction and a thin glass layer to improve the probability of tunneling from Ag crystallites to the Ag grid, contributing to the formation of good ohmic contacts and conductive chains. The hybrid silver powder SH3 gave optimum results as it seems to achieve a balance between the rate of Ag particle sintering and Ag dissolution in the glass during firing.Acknowledgements
The authors are grateful to the financial supports of the National Hi-Tech Research and Development Program (863) Key Project of China (No. 2012AA050301-SQ2011GX01D01292), and Xi'an Industrial Technology Innovation Project-technology transfer promoting program (No. CX1242, CXY1123-5, CX12182-2, CX12182-3, CXY1421 and CXY1511 (9)).Notes and references
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- D. K. Schroder and D. L. Meier, IEEE Trans. Electron Devices, 1984, 31, 637–647 CrossRef.
- C. H. Chen, W. C. Shih, C. Y. Chien, C. H. Shu and C. H. Lai, Sol. Energy Mater. Sol. Cells, 2012, 103, 25–29 CrossRef CAS.
- D. Liang, Y. Kang, Y. Huo, Y. Chen, Y. Cui and J. S. Harris, Nano Lett., 2013, 13, 4850–4856 CrossRef CAS PubMed.
- C. Yang, W. Lin, Z. Li, R. Zhang, W. Wen, B. Gao, G. Chen, P. Gao, M. M. F. Yuen and C. P. Wong, Adv. Funct. Mater., 2011, 21, 4582–4588 CrossRef CAS.
- W. Li, T. Wu, R. B. Jiao, B. P. Zhang, S. Y. Li, Y. Zhou and L. L. Li, Colloids Surf., A, 2015, 466, 132–137 CrossRef CAS.
- A. Kalio, M. Leibinger, A. Filipovic, K. Krüger, M. Glatthaar and J. Wilde, Sol. Energy Mater. Sol. Cells, 2012, 106, 51–54 CrossRef CAS.
- J. T. Wang, L. J. Zhou, H. K. Zhu, R. Yang, Y. Y. Zhou, L. Liu, T. Wang and J. P. Chen, Photonics Res., 2015, 3, 58–62 CrossRef CAS.
- K. Park, D. Seo and J. Lee, Colloids Surf., A, 2008, 313, 351–354 CrossRef.
- G. Guo, W. Gan, F. Xiang, J. Zhang, H. Zhou, H. Liu and J. Luo, J. Mater. Sci.: Mater. Electron., 2010, 22, 527–530 CrossRef.
- Y. N. Ko, H. Y. Koo, J. H. Yi, J. H. Kim and Y. C. Kang, J. Alloys Compd., 2010, 490, 582–588 CrossRef CAS.
- Q. Che, H. Yang, L. Lu and Y. Wang, J. Alloys Compd., 2013, 549, 221–225 CrossRef CAS.
- L. Porter, A. Teicher and D. Meier, Sol. Energy Mater. Sol. Cells, 2002, 73, 209–219 CrossRef CAS.
- S. B. Cho, K. K. Hong, B. M. Chung and J. Y. Huh, in 34th IEEE Photovoltaic Specialists Conference (PVSC), Philadelphia, CA, USA, 2009, pp. 766–769 Search PubMed.
- Y. Ren, Y. Yang, T. Bao, C. Fan and G. Chen, Surf. Interface Anal., 2012, 44, 856–862 CrossRef CAS.
- J. Y. Wu, P. Cao, T. Pan, Y. X. Yang, C. Y. Qiu, C. Tremblay and Y. K. Su, Photonics Res., 2015, 3, 9–14 CrossRef CAS.
- G. J. Zheng, Y. P. Tai, H. Wang and J. T. Bai, J. Mater. Sci.: Mater. Electron., 2014, 25, 3779–3786 CrossRef CAS.
- H. P. Wu, X. J. Wu, M. Y. Ge, G. Q. Zhang, Y. W. Wang and J. Z. Jiang, Compos. Sci. Technol., 2007, 67, 1116–1120 CrossRef CAS.
- H. Yu, L. L. Li and Y. J. Zhang, Scr. Mater., 2012, 66, 931–934 CrossRef CAS.
- M. M. Hilali, K. Nakayashiki, C. Khadilkar, R. C. Reedy, A. Rohatgi, A. Shaikh, S. Kim and S. Sridharan, J. Electrochem. Soc., 2006, 153(1), A5–A11 CrossRef CAS.
- J. Y. Chen, C. K. Huang, W. B. Hung, K. W. Sun and T. M. Chen, Sol. Energy Mater. Sol. Cells, 2014, 120, 168–174 CrossRef CAS.
- L. L. Ye, Z. H. Lai, J. H. Liu and A. Tholen, IEEE Trans. Electron. Packag. Manuf., 1999, 22, 299–302 CrossRef CAS.
- H. H. Lee, K. S. Chou and Z. W. Shih, Int. J. Adhes. Adhes., 2005, 25, 437–441 CrossRef CAS.
- Z. Liu, X. L. Qi and H. Wang, Adv. Powder Technol., 2012, 23, 250–255 CrossRef CAS.
- G. Wang, H. Wang, Y. B. Cui and J. T. Bai, J. Mater. Sci.: Mater. Electron., 2014, 25, 487–494 CrossRef CAS.
- R. X. Li, Y. P. Tai, J. T. Bai and H. Wang, J. Mater. Sci.: Mater. Electron., 2015, 26, 2471–2479 CrossRef CAS.
- Y. P. Tai, G. J. Zheng, H. Y. Wang, H. Wang and J. T. Bai, RSC Adv., 2015, 5, 92515–92521 RSC.
- C. Ballif, D. M. Huljic, G. Willeke and A. Hessler-Wyser, Appl. Phys. Lett., 2003, 82, 1878–1880 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |