Co-supported catalysts on nitrogen and sulfur co-doped vertically-aligned carbon nanotubes for oxygen reduction reaction†
Abstract
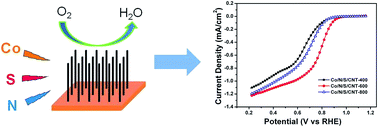
Co supported on nitrogen and sulfur co-doped vertically-aligned carbon nanotubes (Co/NS-CNT) has been fabricated as an efficient electrocatalyst for oxygen reduction reaction (ORR) by a two-step process involving sputtering of cobalt and subsequent annealing in a nitrogen and sulfur-containing atmosphere. The surface morphology, crystal structure and chemical composition of the samples have been investigated. Both cyclic voltammetry (CV) and rotating-disk electrode (RDE) measurements reveal that the annealing temperature has a significant impact on the ORR activity of the catalyst in both alkaline and acid electrolytes. And the best ORR performance is achieved for the catalyst annealed at 600 °C (Co/NS-CNT-600) in 0.1 M KOH solution, which exhibits an onset potential of 0.962 V and an ORR peak potential of 0.803 V. Rotating ring-disk electrode (RRDE) testing in KOH shows an electron transfer number of around 3.7, indicating a four-electron pathway-dominated ORR process. The Co/NS-CNT-600 catalyst also exhibits the best ORR catalytic activity in 0.5 M H2SO4 medium. The excellent ORR activity of the Co/NS-CNT catalyst is attributed to the synergistic effects from N and S co-doping and the increased active sites from metallic cobalt or CoS2.

 Please wait while we load your content...
Please wait while we load your content...