Efficient visible light photocatalytic degradation of 17α-ethinyl estradiol by a multifunctional Ag–AgCl/ZnFe2O4 magnetic nanocomposite†
Akhanda Raj Upreti,
Yi Li*,
Nirina Khadgi,
Saraschandra Naraginti and
Chi Zhang
Key Laboratory of Integrated Regulation and Resource Development of Shallow Lakes, Ministry of Education, College of Environment, Hohai University, Xikang Road #1, Nanjing 210098, P. R. China. E-mail: envly@hhu.edu.cn; akhandaraj@live.com
First published on 24th March 2016
Abstract
The visible light active magnetic photocatalyst ZnFe2O4 was prepared via a facile hydrothermal and deposition–precipitation method using UV irradiation for photoreduction of Ag over AgCl for various durations. The Ag–AgCl/ZnFe2O4 nanocomposite (NC) showed efficient photocatalytic activity for the degradation of 17α-ethinylestradiol (EE2), a recalcitrant endocrine disrupting compound. The pseudo rate constant (k′) of as-synthesized Ag–AgCl/ZnFe2O4 NC photo-reduced for 60 minutes was higher by a factor of 18.3 times compared to the ZnFe2O4 alone. In this Ag–AgCl/ZnFe2O4 NC, plasmon-excited Ag nanoparticles (NP) served as electron-transfer media, while ZnFe2O4 also participated in the charge transfer, besides serving as a support for Ag–AgCl. The electron transfer from ZnFe2O4 to Ag to AgCl in the Ag–AgCl/ZnFe2O4 NC enhances the interfacial charge transfer, and also contributes to the stability of the composite. Further, the composite can be magnetically separated for reuse. Hence, Ag–AgCl/ZnFe2O4 has a good potential in the field of environmental applications.
Introduction
Endocrine disrupting compounds (EDCs) are a structurally diverse class of emerging contaminants occurring especially in wastewater-impacted surface water, groundwater, estuarine water, and drinking water throughout the world.1 EDCs cause various environmental and health problems. They can interfere with hormone systems, resulting in reproductive problems, cancer, hypospadias, miscarriages, endometriosis, and infertility.217α-Ethinylestradiol (EE2, Fig. 1), one of such synthetic steroid estrogen derived from the natural hormone, estradiol (E2) is an orally bio-active estrogen, and is one of the most commonly used medications for humans as well as livestock and aquaculture activity.3,4 EE2 have been reported to have the ability to alter sex determination, delay sexual maturity, and decrease the secondary sexual characteristics of exposed organisms even at a low concentration (ng L−1) by mimicking its natural analogue, 17β-estradiol (E2).4 EE2 is known to enter the aquatic environment via sewage effluents, thereby posing a threat to aquatic wildlife. EE2 has been shown to have estrogenic activity even in the concentration as low as 0.1 ng L−1.5 EE2 has become a widespread problem in the environment because it is a chemically stable emerging contaminant and has a tendency to absorb organic matter, accumulate in sediment and concentrate in biota. Conventional wastewater treatment systems cannot efficiently degrade EE2 as it is resistant to biodegradation.6 Hence, new methods for degradation of recalcitrant pollutants like EE2 from the waste water prior to their release into water bodies is one of the key concerns for water reclamation processes as well as aquatic environment.
 | ||
| Fig. 1 Chemical structure of 17α-ethinylestradiol. Chemical formula: C20H24O2, and exact mass = 296.178. | ||
Advanced oxidation processes (AOPs) have received great attention for water treatment in recent years due to the high efficiency in generating reactive oxidative species (ROS) such as hydroxyl radical, superoxide anion radical and singlet oxygen, which can rapidly oxidize and remove a wide range of organic pollutants from water.6 Heterogeneous photocatalysis is one of the most popular advanced oxidation processes (AOPs) for removal of recalcitrant contaminants.7 The most widely applied photocatalyst in the research of water treatment till date is the Degussa P-25 TiO2,6 extensively used as a standard reference for comparisons of photoactivity under different treatment conditions.8 However, its wide band gap (>3 eV) limits its practicability because it requires UV light for photo activation which inhibits its overall efficiency under natural sunlight as solar spectrum consists of 5% UV, 43% visible and 52% infrared radiation.9 Rigorous researches are underway in search of efficient photocatalysts which are activated by visible light7 as it is one of the attractive approaches for the degradation of recalcitrant EDCs.2
Among various techniques available for the enhancement of visible light active photocatalysis, composite photocatalysts have drawn more attention owing to their significant increase in the photocatalytic activity.10 Formation of heterojunction between two semiconductors allows the interaction of the band structure which effectively prevents the electron–hole recombination and enhance the photocatalytic activity. Zinc ferrite (ZnFe2O4) is one of the most widely used spinel ferrite because of its narrow band gap (∼1.9 eV), low toxicity, environmental benignity and natural abundance.7,11 However, ZnFe2O4 alone cannot be used to degrade pollutants as it has very low quantum yield due to low valence band potential and poor photoelectric conversion property.12
Recently, Ag–AgCl composite has received significant attention as a plasmonic photocatalyst for the photodegradation of organic pollutants.13–18 The high photocatalytic efficiency in their studies was attributed to the Local Surface Plasmon Resonance (LSPR) exhibited by Ag nanoparticles (NPs) in the visible range of the spectrum. Since the surface of AgCl particles is most likely terminated by Cl ions, and is therefore negatively charged, Ag NP deposited on the surface of an AgCl particle should polarize its electron distribution such that the regions of its negative and positive charges are far from and close to the Ag/AgCl interface, respectively.14 AgCl has a wide band gap and so the absorption of visible light by the Ag–AgCl takes place at the Ag NPs. As the surface plasmon state of Ag NPs has the dipolar character, an absorbed photon efficiently separates an electron and a hole such that the electron is transferred to the surface of the NP farthest away from the Ag/AgCl interface while the hole to the surface of the AgCl particle bearing the NP. Several studies19–23 reported increased degradation rate with the presence of Ag due to stronger absorption in the ultraviolet and visible region and enhanced separation of charge by diverting the flow of electrons and holes towards opposite direction.
Plasmonic photocatalysts coupled with semiconductors are one of the most popular combinations in environmental remediation applications. Studies based on ZnFe2O4 and Ag–ZnFe2O4 have been conducted earlier, however, a ternary composite of Ag–AgCl/ZnFe2O4 has not been reported so far. Moreover, the photocatalytic efficiency of the composites with ZnFe2O4 has been mostly studied using the degradation of dyes such as methyl orange, methylene blue, rhodamine B, and in the presence of oxidant, H2O2 in most cases.19,24,25 Very few studies account for the photodegradation of persistent non-dye compound like EE2.
In this work, we prepared Ag–AgCl/ZnFe2O4 composite with different UV irradiation time for Ag loading over AgCl. The as prepared NCs could degrade EE2 efficiently under visible light irradiation and could be magnetically retrieved easily from the photocatalytic system for reuse. We determined the optimal reductive irradiation time for the catalyst based on the degradation efficiency and determined main species involved in the photocatalytic degradation with the help of various scavengers. We also determined hydroxyl radicals (˙OH) and superoxide radical (˙O2−) generation during the photocatalytic reaction. Finally, a plausible photocatalytic mechanism was proposed.
Experimental
Materials and chemicals
Salts of iron(III) (Fe(NO3)3·9H2O) and zinc(II) (Zn(NO3)2·6H2O), H2O2, H2SO4, NaNO3, KMnSO4, cetyltrimethylammonium chloride (CTAC), sodium acetate, isopropanol, potassium iodide (KI), EDTA, potassium dichromate (K2Cr3O7), NaCH3COOH, nitroblue tetrazolium (NBT), terephtalic acid (TA), ascorbic acid, ethylene glycol, ethanol were purchased from Nanjing Chemical Reagent Factory (China). HPLC grade methanol was obtained from Merck Ltd. (Germany). EE2 and Silver nitrate (AgNO3) was purchased from Sigma-Aldrich. All the chemicals were of analytical grade and were used without further purification. Stock solution of EE2 (1000 mg L−1) was prepared by dissolving EE2 in methanol as stock solutions.Synthesis of ZnFe2O4 nanospheres
2 mmol Fe(NO3)3·9H2O and 1 mmol Zn(NO3)2·6H2O were dispersed in 30 mL of ethylene glycol, followed by the addition of 0.8 g of NaCH3COOH under ultrasonic dispersion (30 min) to form clear solution.26 Then the solution was transferred into a Teflon-lined stainless steel autoclave for hydrothermal treatment at 180 °C for 12 hours. After the autoclave was allowed to cool down to room temperature naturally, the precipitate was collected by filtration and washed several times with water and ethanol and dried in an oven at 60 °C for 6 h.Preparation of Ag–AgCl/ZnFe2O4
ZnFe2O4 (0.2 g) and CTAC (0.3 g) were added into 100 mL DI water while stirring at room temperature. After the mixture was stirred for 60 min, AgNO3 (4.0 mL, 0.05 M) was quickly added into the mixture. The resulting solution was further stirred for 60 min and irradiated with an 8 W UV light for 30 min. Finally, the suspension was collected by centrifugation and dried at 80 °C for 8 h and calcined at 300 °C for 3 hours. Similar procedure was followed for the series of composites with different UV irradiation time. The products were referred as Ag–AgCl/ZnFe2O4-30, Ag–AgCl/ZnFe2O4-60 and Ag–AgCl/ZnFe2O4-90 respectively according to 30, 60 and 90 minutes of UV irradiation time.Characterizations
Morphology of the Ag–AgCl/ZnFe2O4 nanocomposites was characterized by transmission electron microscopy (TEM, Zeiss-EM10C) at accelerating voltages of 200 kV. The TEM samples were prepared by dispersing the desired powder in ethanol, and then, dipping carbon coated copper grids into the suspension. X-ray diffraction (XRD) analysis was conducted using a diffractometer (Bruker AXS, Germany) with Cu Kα radiation (λ = 1.54059 Å) at 45 kV and 40 mÅ in the range of 2θ between 20° and 80°. EDAX analysis was carried out by a Hitachi S570 scanning electron microscope equipped with an EDAXPV 9100 energy-dispersion X-ray fluorescence analyzer. Ultraviolet-visible (UV-vis) diffuse reflectance spectroscopy was performed using a SHIMADZU UV-2450 instrument with a collection speed of 40 nm min−1 using BaSO4 as the reference. X-ray photoelectron spectroscopy (XPS) results were obtained with PHI 5000C ESCA with Mg Kα source operating at 14 kV and 25 mA. All the binding energies were referenced to the contaminant C 1s peak at 284.6 eV of the surface adventitious carbon.Photocatalytic degradation experiment
Photocatalytic activity of the Ag–AgCl/ZnFe2O4 nano-composites was evaluated by degradation of 17α-ethinyl estradiol (EE2) as a representative of resistant organic pollutant in an aqueous solution. Prior to irradiation, 50 mg of the Ag–AgCl/ZnFe2O4 composite was dispersed in 100 mL of 5 mg L−1 EE2 aqueous solution in a beaker and continuously stirred magnetically for 60 minutes to obtain the absorption–desorption equilibrium. Then under continuous stirring the solution was irradiated by a 300 W Xe arc lamp equipped with wavelength cut off filter to eliminate the radiation with λ ≤ 420 nm. At given time intervals, 3 mL of the aliquots were withdrawn until 4 hours using a syringe and filtered using a 0.4 μm Millipore membrane filter. The reaction setup is shown in Fig. S1.†Analytical methods
The concentration of remnant EE2 was determined by high-performance liquid chromatography (HPLC) technique. The fluorescence detector of Agilent 1260 series with Eclipse XDB-C18 (5 μm) reverse phase column (4.6 × 150 mm) was used for the reverse-phase separation. The fluorescence detector with an excitation wavelength of 280 nm and an emission wavelength of 310 nm was used.27 The eluent consisted of a mixture of water and methanol (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 by volume), injection volume and the flow rate were set to 10 μL and 1 mL min−1 respectively for 6 minutes. The peak was observed at 4.5 minutes. The detection limits (MDL) was 0.01 mg L−1 as inferred by the lowest standard solution detected. EE2 photocatalytic degradation products were separated and identified by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Agilent 1290 Infinity Binary LC system, Agilent 6460 Triple Quadrupole LC-MS/MS system employing the Zorbax eclipse plus c18 column; rapid resolution, 2.1 × 50 mm, 1.8 micron). Details of the HPLC-MS/MS methods are provided in ESI.†
70 by volume), injection volume and the flow rate were set to 10 μL and 1 mL min−1 respectively for 6 minutes. The peak was observed at 4.5 minutes. The detection limits (MDL) was 0.01 mg L−1 as inferred by the lowest standard solution detected. EE2 photocatalytic degradation products were separated and identified by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Agilent 1290 Infinity Binary LC system, Agilent 6460 Triple Quadrupole LC-MS/MS system employing the Zorbax eclipse plus c18 column; rapid resolution, 2.1 × 50 mm, 1.8 micron). Details of the HPLC-MS/MS methods are provided in ESI.†
Active species scavenging experiments
To investigate the main active species for the photocatalytic reaction and degradation process, a series of scavenging experiments were conducted by adding ascorbic acid as ˙O2− scavenger,28 EDTA as h+ scavenger,29 potassium dichromate as e− scavenger,30 isopropanol as ˙OH scavenger,31 and potassium iodide (KI) as both ˙OH and h+ scavenger28,29 during the photocatalytic reaction experiments.Determination of ˙OH and ˙O2− radicals
Terepthalic acid (TA) and nitro blue tetrazolium (NBT) were used in order to determine the generation of the main active species, such as hydroxyl radicals (˙OH) and superoxide radical (˙O2−) produced during the photoreaction process respectively. To determine the production of ˙OH radicals 25 mg of the photocatalyst was added to a 50 mL mixture of TA solution (3 mmol) and NaOH (10 mmol). The production of ˙OH radicals under visible light irradiation was monitored by HITACHI F-7000 Fluorescence Spectrophotometer. TA readily reacts with ˙OH hydroxyl radicals to generate 2-hydroxyterephthalic acid (TAOH) which emits fluorescence around 426 nm on excitation of its own 312 nm absorption band. The increase in photoluminescent intensity of TAOH with time should be directly proportional to the ˙OH radicals generation. Similarly NBT (10 ppm) was used to detect the amount of ˙O2− radicals generation from 25 mg of catalyst. The production of superoxide radicals in suspension was quantitatively analyzed by monitoring the concentration of NBT in the supernatant solutions through measuring the absorbance at 259 nm, λmax in a UV-vis-spectrophotometer (Thermo Scientific Orion aqua mate 8000).Results and discussion
Characterizations
The XRD patterns of ZnFe2O4 and Ag–AgCl/ZnFe2O4 nanocomposites are shown in Fig. 2. The intensity of the ZnFe2O4 peak decreased after the addition of Ag–AgCl over the surface of ZnFe2O4.As seen in the XRD plot, the as-prepared sample contains cubic phase AgCl and metallic Ag. The diffraction peaks at 2θ of 27.6 (111), 32.1 (200), 46.1 (220), 54.7 (311), 57.3 (222), 67.3 (400), 74.6 (311) and 76.8 (420) are very close to the cubic phase of AgCl with lattice constant a = 0.5549 nm (JCPDS 31-1238). The peaks at 2θ of 38.2 (111), 44.3 (200), and 64.4 (220) can be assigned to the cubic phase of Ag with lattice constant a = 0.4086 nm (JCPDS 65-2871). The peaks at 2θ = 30.1 (220), 35.3 (311), 43.0 (400), 53.5 (422), 56.3 (511), and 62.4 (440) can be assigned to spinel ZnFe2O4 (JCPDS 22-1012). The relatively lower intensity for the diffraction peaks of Ag should be due to the low content and/or low degree of crystallinity of the metallic Ag in the sample (Hou et al. 2013; Shu et al. 2014). There were no impurity phases in the XRD of Ag–AgCl/ZnFe2O4-60 which indicates the composite sample contained Ag0, AgCl, and ZnFe2O4.
Fig. 3 shows the TEM images of Ag–AgCl/ZnFe2O4-60 nano-composite. The composite contains slightly aggregated tiny nanocrystals with more or less uniform grain sizes (Fig. 3A) ranging from 10–20 nm (Fig. 3B). The 0.32 nm lattice fringe, corresponding to (111) plane of AgCl nanoparticles, is simultaneously observed. Further, in the Fig. 3C, the interlayer spacing d = 0.23 nm corresponding to the (111) cubic plane of Ag could be seen. The distance between one set of fringes is 0.49 nm, which is close to the (111) plane of spinel ZnFe2O4 NPs. The EDAX spectrum of the composite also confirms the formation of composite (Fig. 3D).
 | ||
| Fig. 3 TEM images of Ag–AgCl/ZnFe2O4-60 at (A) 100 nm, (B) 20 nm, (C) HRTEM, (D) EDAX spectrum of Ag–AgCl/ZnFe2O4-60. | ||
X-ray photoelectron spectroscopy (XPS) was performed to further investigate the surface chemical composition and chemical states of the as-prepared samples (Fig. 4). The survey XPS spectrum (Fig. 4A) of the as-prepared NC confirmed the main ingredient elements Zn, Fe, O, Ag and Cl, which agreed well with the results of elemental mapping analysis (Fig. 3D). The C 1s and O 1s peaks are probably due to the adventitious hydrocarbon from the XPS instrument itself. The spectrum of Ag 3d consists of two main peaks at 367.2 eV and 373.2 eV (Fig. 4B), which are ascribed to Ag 3d5/2 and Ag 3d3/2, respectively.17 The peaks of Ag 3d5/2 and Ag 3d3/2 could be further divided into different peaks at 367.2 eV, 368.2 eV and 373.2 eV, 374.2 eV, respectively. The peaks at 367.2 eV and 373.2 eV could be attributed to the peaks of Ag 3d5/2 and Ag 3d3/2 of Ag+ in AgCl, whereas the peaks at 368.2 and 374.2 eV could be ascribed to the peaks of Ag 3d5/2 and Ag 3d3/2 metallic Ag0 respectively. The peak around 198 and 199 eV can be assigned to Cl 2p of the AgCl nanoparticles (Fig. S3†). All the XPS binding energies accord well with the earlier studies.20,33
Photocatalytic activity
The photocatalytic activity of Ag–AgCl/ZnFe2O4 was evaluated by performing photodegradation of EE2 under visible light. No significant EE2 degradation occurred in the absence of catalyst, indicating the stability and slow photolysis of EE2. Before the test of as-prepared NCs, ZnFe2O4 was tested for the reference and only about 30% of EE2 was degraded in 240 minutes. Among the three kinds of Ag–AgCl/ZnFe2O4 NCs, Ag–AgCl/ZnFe2O4-60 showed the highest photocatalytic activity followed by Ag–AgCl/ZnFe2O4-30 (Fig. 5A). This least photocatalytic activity for ZnFe2O4 compared to Ag–AgCl/ZnFe2O4 NCs can be attributed to the lower valence band potential and poor photoelectric conversion property of ZnFe2O4.12 The enhancement in photocatalytic efficiency in Ag–AgCl/ZnFe2O4 samples might also be attributed to the surface plasmon resonance effect induced by Ag NPs,34 leading to more absorption of photon and increase in charge separation efficiency and the promotion of the charge-transfer to the reaction substrates.35 | ||
| Fig. 5 (A) Photocatalytic degradation of EE2 and (B) rate constants for the degradation of EE2 by different NCs under visible light irradiation. | ||
The reason for the different performance shown by different composite might be explained by the difference in irradiation time. Different photoreduction time in the preparation process can result in different amount of Ag0 or different NP size on the surface of AgCl/ZnFe2O4. According to earlier studies,9,20 the appropriate ratio of Ag0/Ag+ is essential for effective charge separation, which plays a key role for the efficient photocatalysis. If the ratio is high, there is the formation of Ag cluster, which then turns out to be the recombination center for photoinduced carriers and ultimately counteracts the activity improvement.20 The ratios of Ag0/AgCl was calculated using the classical chemical analysis method17,20 using atomic absorption spectrophotometry. The ratio of Ag0 to AgCl was found out to be 3.2%, 5.1% and 6.9% for Ag–AgCl/ZnFe2O4-30, Ag–AgCl/ZnFe2O4-60 and Ag–AgCl/ZnFe2O4-90 respectively. The optimum ratio was achieved in Ag/AgCl/ZnFe2O4-60 as it exhibited the best photocatalytic activity followed by Ag/AgCl/ZnFe2O4-30 and Ag/AgCl/ZnFe2O4-90.
The DRS results (Fig. S2†) demonstrated that the absorbance of Ag–AgCl/ZnFe2O4 increased with the increase of photoreduction time from 30 min to 60 min, which tended to increase the surface redox potentials and to prolong the carrier lifetime. The series of Ag–AgCl/ZnFe2O4 composites exhibited stronger absorption in visible light region and broad absorption in the 400–750 nm region of visible light with absorption tail extending into infra-red region which can be attributed to the surface plasmon resonance of Ag NPs produced by the photoreduction of AgCl19 and the interaction between Ag and AgCl. Ag–AgCl/ZnFe2O4-60 showed highest absorption in the visible region. The strong absorption in the UV region is characteristic to semiconductor materials. This corresponded with the increase in photoactivity when irradiated until 60 minutes and declined as the irradiation time was prolonged to 90 min. This decline might be the result of excessive metallic Ag0 being produced by the photoreduction of AgCl, probably giving rise to particle agglomeration of Ag0 with decline in nanocrystal heterojunction, which causes a decrease in the photocatalytic activities.36 Further, the decrease in activity with reaction time might also be attributed to the deposition of metallic silver in excess on the catalyst surface, which blocks light absorption and obstructs active sites.37
Considering that the degradation of EE2 follows the pseudo first-order kinetics,20 the rate constant (k′) for the degradation of EE2 over Ag–AgCl/ZnFe2O4-60 was calculated to be 0.0183 min−1 (Fig. 5B) while that for Ag–AgCl/ZnFe2O4-30, Ag–AgCl/ZnFe2O4-90 and bare ZnFe2O4 were 0.0036 min−1, 0.0023 min−1 and 0.001 min−1 respectively. The turnover number (TON) for the photodegradation of EE2 at 51.5% (TON = 14.66) and 68.8% (TON = 19.61) demonstrate that a photoreaction occurring on the surface of the photocatalyst is truly photocatalytic.38 For the turnover frequency (TOF), if the time is calculated up to, for instance, nearly 90% consumption of the reactant, the result becomes significantly different from considering up to 30% consumption, as is shown in Table S1† since the TOF is usually a function of the reactant concentration.39
Active species scavenging experiment
A series of scavenging experiments were conducted to identify the main active species for the photocatalytic reaction and degradation process, using ascorbic acid as ˙O2− scavenger, EDTA as h+ scavenger, potassium dichromate as e− scavenger, isopropanol as ˙OH scavenger, and potassium iodide (KI) as both ˙OH and h+ scavenger (Fig. 6). After the addition of the scavengers, the decrease in photocatalytic degradation followed the order ascorbic acid > isopropanol > EDTA > potassium iodide > potassium dichromate > no scavenger, when observed for 240 minutes of photoreaction time. In the N2-saturated solution, EE2 degradation over Ag–AgCl/ZnFe2O4 was significantly inhibited. The degradation of EE2 was most interfered with the addition of ascorbic acid, which shows that ˙O2− is the primary species in the photocatalytic process. Further, the introduction of isopropanol which scavenges ˙OH and; KI, which reacts with both h+ and ˙OH also restrained the degradation of EE2 significantly, implying the superoxide radicals followed by ˙OH and h+ are the main active oxidative species of the Ag–AgCl/ZnFe2O4 photocatalyst. Addition of potassium dichromate caused the least decline in photodegradation indicating that role of electrons in direct reduction reaction is minimal.Determination of ˙O2− radicals
The primary active species that are formed as a result of photoexcitation of semiconductors are ˙OH hydroxyl and ˙O2− superoxide radicals. In order to estimate the rate of formation of active ˙O2− superoxide radicals under visible light irradiation, we employed NBT method in which NBT can be specifically reacted with ˙O2− forming insoluble purple formazan as precipitate over the catalyst.40,41 The generation of ˙O2− can be quantitatively evaluated from the decrease of NBT concentration. Fig. 7 showed decrease in absorbance at around 259 nm with irradiation time indicating increasing concentration of active superoxide ˙O2− radical species. The decrease in NBT concentration with irradiation time is the result of chemical reaction between ˙O2− and NBT.40,42Determination of ˙OH radicals
The production of ˙OH radical during photodegradation experiment was detected by the emission of photoluminescence (PL) using terepthalic acid (TA) as a probe. Fig. 8 shows the ˙OH-trapping PL spectra of Ag–AgCl/ZnFe2O4 in TA solution at room temperature under visible light irradiation. When the photocatalytic system was irradiated by the visible light, the photoluminescence emission peak of TAOH (around 426 nm) was continuously enhanced indicating the generation of ˙OH in the Ag–AgCl/ZnFe2O4 photocatalytic system under visible light irradiation. The generation of ˙OH plays an important role in the superior visible photocatalytic activity of the Ag–AgCl/ZnFe2O4 system. | ||
| Fig. 8 ˙OH-trapping PL spectra of Ag–AgCl/ZnFe2O4 in solution of terephthalic acid at room temperature. | ||
Proposed mechanism of photocatalytic degradation
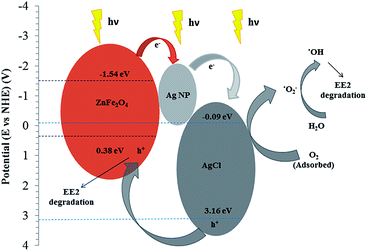
On the basis of the experimental results and the literatures,12,14,17,19,24,32,43,44 the photocatalytic degradation mechanism of EE2 by the Ag–AgCl/ZnFe2O4 nanocomposite under visible light irradiation was proposed. ZnFe2O4 can be excited by the visible light and thus the electron–hole pairs are generated.32 The photogenerated electrons in the CB bottom of ZnFe2O4 can move into the Ag NPs and might immediately transfer to the CB of AgCl. The plasmon-induced electrons on Ag NPs might flow to the conduction band (CB) of AgCl rather than ZnFe2O4 because of the less negative CB bottom of AgCl (−0.09 V vs. NHE)13 as compared to that of ZnFe2O4 (−1.54 V vs. NHE).45 The CB electrons on AgCl would be scavenged by dissolved oxygen and transformed into super oxygen anionic free radicals (˙O2−),14 which was found to be the main active species (Fig. 9) in the photocatalytic system. The photogenerated valence band (VB) holes in the AgCl transfer more readily to the VB of ZnFe2O4.36,45 Further, the reactive holes at the top of the VB of ZnFe2O4, cannot oxidize surface adsorbed H2O to ˙OH because the top of the VB of ZnFe2O4 (0.38 V vs. NHE)45 is less positive than that of E(˙OH/H2O = 2.8 V vs. NHE)25 and therefore h+ might be directly involved in the oxidation of EE2.36 Some of the photogenerated electrons in the CB of Ag NPs can be trapped by dissolved O2 in the solution to yield ˙O2− which could be further utilized for the degradation and mineralization of the absorbed EE2.14The electron transfer from ZnFe2O4 to Ag NPs to AgCl in the Ag–AgCl/ZnFe2O4 composite enhances the interfacial charge transfer. The efficient transfer of the charge carriers as discussed above prolong their lifetime and suppress the unfavourable recombination and enhances photocatalytic activity and stability of the composite.13
Photodegradation pathway of EE2
To elucidate the structures of the intermediates formed during photocatalytic degradation of EE2, the samples taken after a reaction time of 120 minutes and 240 minutes were subjected to LC/MS analysis. Based on the LC-MS and several earlier literatures.3,5,31,46 The mass spectral pattern of samples, the molecular weight of each detected by-products as well as the major ions in their ion spray mass spectra are shown in Fig. S4 and S5.† The results show that EE2 was first oxidized into semiquinone, which were finally degraded into organic acids and different small molecules as shown in Fig. 10. The scavenging experiments (Fig. 6) suggested that ˙O2− radicals were the main reactive oxidants in the EE2 photodegradation in the presence of Ag–AgCl/ZnFe2O4 catalyst. Therefore, it is worth mentioning that further attack of superoxide radicals on cationic radical (compound A) at the position 10 of the aromatic ring results in a multistep pathway of degradation.46Magnetic separation and recyclability of the catalyst
The long term efficacy of a catalyst depends on its stability. Therefore, the stability of the as synthesized photocatalyst was studied by performing the recycle tests for five times on Ag–AgCl/ZnFe2O4-60 NC and the results are shown in Fig. 11A. The activity of the photocatalyst under visible light did not change significantly (kept over 90% of its original activity) which indicate that Ag–AgCl/ZnFe2O4 is an active and stable visible-light-driven photocatalyst. Most of the currently available photocatalysts involve a difficult catalyst-recovery steps due to their nano size, which significantly increase the practical running cost.25 However, in this study the Ag–AgCl/ZnFe2O4 NC could be easily separated from the reaction system with an external magnetic field in less than 2 minutes (Fig. 11B). It could be readily re-dispersed into water after removing the magnetic field. This magnetically responsive photocatalyst has key benefit over the conventional photocatalysts, as it can be recovered and reused easily, making it reasonably more practicable and thus poses lower threat of secondary pollution by composite itself to the environment.Conclusions
In this study, we outlined facile methods to fabricate Ag–AgCl immobilized on ZnFe2O4 nanospheres. The Ag–AgCl/ZnFe2O4 composite was able to degrade EE2 within 240 minutes without any additional oxidant. The experimental results indicated ZnFe2O4, Ag and AgCl work synergistically to enhance the photocatalysis process. The plasmonic property of Ag NPs enhanced the photocatalytic capability of ZnFe2O4. Based on various active species scavenging experiments, determination of ˙O2− and ˙OH and band-gap calculation, a possible mechanism for the enhanced photocatalytic activity was proposed, which is ascribed to improved absorptivity, broadened visible-light absorption, effective electron–hole pair generation, efficient charge separation and transfer decreasing their recombination rate. Furthermore, the photocatalyst could be conveniently separated from the suspension by applying an external magnetic field and reused with persistent performance which is very important from economic and environmental point of view to avoid secondary pollution. Simple and cheap synthesis methods as used in this study bring photocatalysis closer to being a viable water treatment option.Acknowledgements
The study was financially supported by the National Natural Science Foundation of China (No. 51322901 and No. 51479066), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 51421006), and Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- S. K. Bhunia and N. R. Jana, ACS Appl. Mater. Interfaces, 2014, 6, 20085–20092 CAS.
- Z. Frontistis, C. Drosou, K. Tyrovola, D. Mantzavinos, D. Fatta-Kassinos, D. Venieri and N. P. Xekoukoulotakis, Ind. Eng. Chem. Res., 2012, 51, 16552–16563 CrossRef CAS.
- A. Z. Aris, A. S. Shamsuddin and S. M. Praveena, Environ. Int., 2014, 69, 104–119 CrossRef CAS PubMed.
- X. Zhang, P. Chen, F. Wu, N. Deng, J. Liu and T. Fang, J. Hazard. Mater., 2006, 133, 291–298 CrossRef CAS PubMed.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- E. Casbeer, V. K. Sharma and X. Li, Sep. Purif. Technol., 2012, 87, 1–14 CrossRef CAS.
- A. Di Paola, E. García-López, G. Marcì and L. Palmisano, J. Hazard. Mater., 2012, 3, 211–212 Search PubMed.
- R. Adhikari, G. Gyawali, T. Sekino and S. Wohn Lee, J. Solid State Chem., 2013, 197, 560–565 CrossRef CAS.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falarasd, A. G. Kontosd, P. S. M. Dunlope, J. W. J. Hamiltone, J. A. Byrne, K. O'Sheaf, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS.
- A. Meidanchi and O. Akhavan, Carbon, 2014, 69, 230–238 CrossRef CAS.
- J. Xie, Q. Wu and D. Zhao, Carbon, 2012, 50, 800–807 CrossRef CAS.
- J. Ke, C. Niu, J. Zhang and G. Zeng, J. Mol. Catal. A: Chem., 2014, 395, 276–282 CrossRef CAS.
- P. Wang, B. Huang, X. Qin, X. Zhang, Y. Dai, J. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931–7933 CrossRef CAS PubMed.
- D. Wang, Y. Li, G. Li, C. Wang, P. Wang, W. Zhang and Q. Wang, J. Hazard. Mater., 2015, 285, 277–284 CrossRef CAS PubMed.
- Y. Tang, Z. Jiang, G. Xing, A. Li, P. D. Kanhere, T. C. Sum, S. Li, X. Chen, Z. Dong and Z. Chen, Adv. Funct. Mater., 2013, 23, 2932–2940 CrossRef CAS.
- J.-F. Guo, B. Ma, A. Yin, K. Fan and W. L. Dai, J. Hazard. Mater., 2012, 211–212, 77–82 CrossRef CAS PubMed.
- C. An, X. Ming, J. Wang and S. Wang, J. Mater. Chem., 2012, 22, 5171–5176 RSC.
- X. Cao, L. Gu, X. Lan, C. Zhao, D. Yao and W. Sheng, Mater. Chem. Phys., 2007, 106, 175–180 CrossRef CAS.
- J.-F. Guo, B. Ma, A. Yin, K. Fan and W.-L. Dai, Appl. Catal., B, 2011, 101, 580–586 CrossRef CAS.
- W. Tang, Y. Su, X. Wang, Q. Li, S. Gaoa and J. K. Shang, RSC Adv., 2014, 4, 30090 RSC.
- J. Hu, J. Ma, L. Wang, H. Huang and L. Ma, Powder Technol., 2014, 254, 556–562 CrossRef CAS.
- J. Shu, Z. Wang, G. Xia, Y. Zheng, L. Yang and W. Zhang, Chem. Eng. J., 2014, 252, 374–381 CrossRef CAS.
- M. Su, C. He, V. K. Sharma, M. Abou Asi, D. Xia, X. Li, H. Deng and Y. Xiong, J. Hazard. Mater., 2012, 211–212, 95–103 CrossRef CAS PubMed.
- W. Wu, C. Jiang and V. A. L. Roy, Nanoscale, 2015, 7, 38–58 CAS.
- P. Guo, L. Cui, Y. Wang, M. Lv, B. Wang and X. S. Zhao, Langmuir, 2013, 29, 8997–9003 CrossRef CAS PubMed.
- Y. Yoon, P. Westerhoff, S. a. Snyder and M. Esparza, Water Res., 2003, 37, 3530–3537 CrossRef CAS PubMed.
- S. Song, L. Xu, Z. He, H. Ying, J. Chen, X. Xi and B. Yan, J. Hazard. Mater., 2008, 152, 1301–1308 CrossRef CAS PubMed.
- Y. Fu, H. Chen, X. Sun and X. Wang, Appl. Catal., B, 2012, 111–112, 280–287 CrossRef CAS.
- L. S. Zhang, K. H. Wong, H. Y. Yip, C. Hu, J. C. Yu, C. Y. Chan and P. K. Wong, Environ. Sci. Technol., 2010, 44, 1392–1398 CrossRef CAS PubMed.
- Z. Pan, E. Stemmler, H. J. Cho, W. Fan, L. LeBlanc, H. H. Patterson and A. Amirbahman, J. Hazard. Mater., 2014, 279, 17–25 CrossRef CAS PubMed.
- Y. Hou, X. Li, Q. Zhao and G. Chen, Appl. Catal., B, 2013, 142–143, 80–88 CrossRef CAS.
- X. Li, Y. Hou, Q. Zhao, W. Teng, X. Hu and G. Chen, Chemosphere, 2011, 82, 581–586 CrossRef CAS PubMed.
- D. Wang, Y. Li, G. L. Puma, C. Wang, P. Wang, W. Zhang and Q. Wang, Chem. Commun., 2013, 49, 10367–10369 RSC.
- D. Zhao, Y. Xiao, X. Wang, Q. Gao and M. Cao, Nano Energy, 2014, 7, 124–133 CrossRef CAS.
- X. Li, D. Tang, F. Tang, Y. Zhu, C. He, M. Liu, C. Lin and Y. Liu, Mater. Res. Bull., 2014, 56, 125–133 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- A. V. Emeline, A. V. Panasuk, N. Sheremetyeva and N. J. Serpone, J. Mater. Chem. B, 2005, 109, 2785–2792 CAS.
- S. Kozuch and J. M. L. Martin, ACS Catal., 2012, 2, 2787–2794 CrossRef CAS.
- T. Hirakawa and Y. Nosaka, Langmuir, 2002, 18(8), 3247–3254 CrossRef CAS.
- S. Uma, J. Singh and V. Thakral, Inorg. Chem., 2009, 48, 11624–11630 CrossRef CAS PubMed.
- Z. Chen, D. Li, W. Zhang, Y. Shao, T. Chen, M. Sun and X. Fu, J. Phys. Chem. C, 2009, 113, 4433–4440 CAS.
- S. Glaus and G. Calzaferri, Photochem. Photobiol. Sci., 2003, 2, 398–401 CAS.
- M. R. Hoffmann, M. R. Hoffmann, S. T. Martin, S. T. Martin, W. Choi, W. Choi, D. W. Bahnemannt and D. W. Bahnemannt, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- S. Boumaza, A. Boudjemaa, A. Bouguelia, R. Bouarab and M. Trari, Appl. Energy, 2010, 87, 2230–2236 CrossRef CAS.
- Y. Ohko, K. I. Iuchi, C. Niwa, T. Tatsuma, T. Nakashima, T. Iguchi, Y. Kubota and A. Fujishima, Environ. Sci. Technol., 2002, 36, 4175–4181 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup of the photocatalytic degradation, UV-vis absorbance spectra of the composites, XPS spectra of Cl 2p binding energy, LC-MS study of EE2: mass fragmentation patterns, calculation of TON and TOF. See DOI: 10.1039/c6ra00707d |
| This journal is © The Royal Society of Chemistry 2016 |