ZLD1122, a novel EZH2 and EZH1 small molecular inhibitor, blocks H3K27 methylation and diffuse large B cell lymphoma cell growth
Abstract
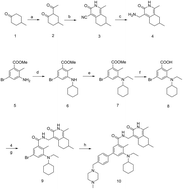
The histone methyltransferase enhancer of zeste homolog 2 (EZH2) has been reported to be overexpressed in a variety of cancers and is associated with tumor malignancy. This is mainly because EZH2 catalyzes the hypertrimethylation of histone 3 at lysine 27 (H3K27) at the promoter of target genes, leading to the silencing of downstream tumor suppressor genes. Hence, blocking its catalytic function may be a therapeutic strategy for the treatment of tumors which over-express or have a gain-of-function mutation in EZH2, such as lymphomas. Here, we reported a novel, selective, small-molecule inhibitor of EZH2 and EZH1 synthesized by us, ZLD1122, which inhibited both EZH1 and wild type and mutant EZH2 activities with nanomolar potency. ZLD1122 significantly inhibited intracellular H3K27 trimethylation without affecting the levels of H3, H3K9me3, and H3K4me3, indicating its selective inhibition of polycomb repressive complex 2 (PRC2) methyl catalytic function. Moreover, ZLD1122 induced G0/G1 phase arrest in diffuse large B cell lymphoma (DLBCL) cells in a dose-dependent manner via downregulation of cyclinE and CDK4 as well as upregulation of p21 and cyclinD1. Furthermore, it induced apoptosis and loss of mitochondrial membrane potential (Δψm), and elevated the levels of cleaved caspase-9 in Su-DHL-6 and Pfeiffer cells, suggesting that ZLD1122 suppresses the viability of DLBCL cells by inducing caspase-mediated intrinsic apoptosis. Taken together, these data demonstrated that ZLD1122, owing to its pharmacologically inhibitory activity against EZH2, could be a promising agent for the treatment of lymphomas with EZH2 gain-of-function mutations.

 Please wait while we load your content...
Please wait while we load your content...