Synthesis and antimalarial activity of novel bicyclic and tricyclic aza-peroxides†
Abstract
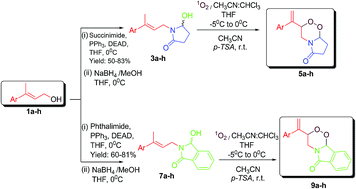
For the first time, novel bicyclic 5a–h as well as tricyclic 9a–h aza-peroxides were synthesized using a 1O2-mediated photo-oxygenation methodology as a key step in 52–71% yields in which one of the oxygen atoms of the 1,2,4-trioxane ring has been replaced by a nitrogen atom. The methodology is simple and is an efficient way to access 1,2-dioxa-4-aza six membered ring compounds. All these compounds were assessed for their in vitro antimalarial activity against Plasmodium falciparum. Compound 9a, 9c and 9f, the most active compound of the series, showed IC50 values of 9.43, 8.83 and 5.63 ng mL−1, respectively which were found to be comparable to that of the antimalarial drug chloroquine (IC50 value 5.2 ng mL−1). Compound 9f, the most active compound found in in vitro studies, provided 40% protection to infected mice at the dose of 96 mg kg−1 × 4 days when screened for its antimalarial activity in vivo against multidrug-resistant Plasmodium yoelii nigeriensis in Swiss mice by the oral route. In this assay, β-arteether and chloroquine, showed 100% suppression of parasitaemia on day 4 and provided 100% and 80% protection, respectively, to the infected mice.

 Please wait while we load your content...
Please wait while we load your content...