Solution combustion synthesis and physico-chemical properties of ultrafine CeO2 nanoparticles and their photocatalytic activity
Abstract
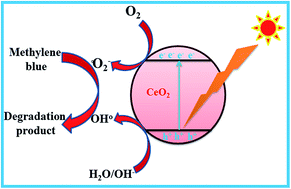
Ultrafine cerium oxide (CeO2) nanoparticles have been confirmed to be capable photocatalysts for environmental remediation because of their strong redox ability, long-term stability, nontoxicity, and cost effectiveness. CeO2 nanoparticles were successfully synthesized by a solution combustion method using cerium nitrate and urea. The physical, chemical, thermal and optical properties of the as-prepared samples were characterized using various analytical techniques. X-ray diffraction data confirm that the synthesized nanocrystalline CeO2 samples have cubic structure with an average grain size of 10, 8 and 19 nm corresponding to as-prepared, 300 and 600 °C annealed samples, respectively. The HRTEM results confirmed that the synthesized nanoparticles exhibit good polycrystalline nature with spherical ultrafine nanoparticles having size in the range of ∼9 nm. The UV/vis spectrum shows a maximum absorption at 294 nm and the band gap of CeO2 was tuned by adjusting the annealing temperature. In the PL spectra, a strong and broad emission band was observed at 425 nm due to the presence of a blue shift in the visible region. The photocatalytic activities of annealed CeO2 nanoparticles were investigated by photodegradation of methylene blue (MB) under UV light irradiation. It was found that the ultra-fine CeO2 nanoparticles exhibited better photocatalytic activity than that of already available commercial photocatalysts. Based on the investigation, these ultra-fine CeO2 nanoparticles possess adaptable potential applications for wastewater purification.

 Please wait while we load your content...
Please wait while we load your content...