Rheological, morphological and mechanical investigations on ethylene octene copolymer toughened polypropylene prepared by continuous electron induced reactive processing
Abstract
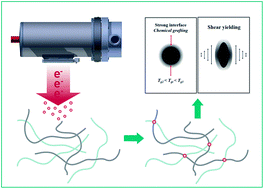
We prepared ethylene octene copolymer (EOC) toughened polypropylene (PP) by continuous electron induced reactive processing (EIReP) as well as investigated its rheological, morphological and mechanical properties. A time temperature superposition (TTS) method was utilized in order to investigate the specific thermo-rheological behavior of these PP/EOC blends. At a low concentration of EOC, a reduced blend viscosity was found. In contrast, the blend viscosity increases at higher concentrations of EOC in comparison to virgin PP. The differences in the molecular architecture of EOC and PP as well as the specific interfacial properties of the PP/EOC blend lead to this behavior. In addition, EIReP modified samples showed lower viscosity than their non-modified counterparts. This reduction of the absolute value of complex viscosity was related to the degradation of the PP phase. In EIReP modified samples an enhancement of the storage modulus at low frequencies was attributed to grafting at the interface and crosslinking of the dispersed EOC phases. All investigations of the thermo-rheological properties showed that TTS holds for the EIReP modified and non-modified blends. Using SEM micrographs, the effect of EIReP and blend ratio on the microstructure of toughened PP was studied. Finally, we discussed the origins of the differences of EOC toughened PP in tensile and impact experiments as well as proposed a micro-mechanism based on the investigation of thermal properties and considering molecular effects of EIReP on polymers.

 Please wait while we load your content...
Please wait while we load your content...