Ultra-small Sn2S3 porous nano-particles: an excellent photo-catalyst in the reduction of aqueous Cr(VI) under visible light irradiation†
Ziwei Wang,
Ziyu Wang,
Dameng Wang and
Ming Chen*
School of Physics, Shandong University, Jinan 250100, China. E-mail: chenming@sdu.edu.cn
First published on 25th January 2016
Abstract
We present the direct conversion of bulk Sn metal to sub-10 nm Sn2S3 porous nano-particles based on pulsed-laser ablation of a Sn target in solution. The as-prepared ultra-small porous structures exhibit superior photocatalytic activity and excellent stability in the reduction of aqueous Cr(VI) under visible light irradiation.
Most recently, photocatalytic reduction of aqueous hexavalent chromium Cr(VI) to Cr3+ under visible light irradiation has attracted tremendous interest, since the Cr(VI) ions in liquid originated from the leather tanning, electroplating, or dyeing industries are highly toxic (about 100 times higher than Cr3+) and are confirmed carcinogens for bladder, liver, kidney, skin cancer, etc.1–9 To achieve visible light-driven photo-catalysts with high-performance for the reduction of Cr(VI), several hybrid multi-inorganic nano-materials with various morphologies have been developed.2–4,6,10 It is believed that the semiconducting metal sulfides are promising photo-catalysts since they are highly efficient, and cost-effective, without discharging any perilous chemicals.3 Recent reports of SnS2 nano-flowers, SnS2/TiO2 hybrid nano-spheres, and SnS2/SnO2 nano-heterojunctions have shown them to be efficient photo-catalysts for the reduction of aqueous Cr(VI). These composites possess low toxicity, good chemical stability and the unique ability to harvest light in the visible region.2,3,7,9 In addition to the compositions, the nano-size and porous shaped structures play important roles in photo-catalytic performance (the activity, stability, etc.). However, these previous reports were mainly focused the synthesis of large-sized (tens to hundreds nanometers) particles with a narrow structure controllability by reduction of the metal precursors in the presence of stabilizing agent. The major drawback is the requiring strict condition for the simultaneous and uniform reduction to occur at high temperature. To further significantly improve the photo-catalytic activity of tin–sulfides material, ultra-small porous nano-structure with excellent-high surface area is strongly required. It has been demonstrated to exhibit remarkable electron transport, efficient localized surface plasmon resonance, and super photo-catalytic activity.8,9
Herein, for the first time, we report the successful synthesize of the sub-10 nm Sn2S3 porous nano-particles with by pulsed laser ablation of Sn target in activated liquid. The liquid solution contains deionized water, hexadecyl trimethyl ammonium bromide (CTAB), thioacetamide (TAA) and hydrochloric acid (HCl), which can be adjusted to obtain desirable porous nano-structures. The direct conversion of bulk target to ultra-small Sn2S3 porous nano-particles is strongly depends on the unique hot Sn plasma generated by laser ablation, and highly non-equilibrium nucleation process including the ultra-rapid acid etching between Sn/S and HCl solution. The obtained ultra-small Sn2S3 nano-particles exhibit superior improved photo-catalytic activity and excellent stability in photo-catalytic reduction of aqueous Cr(VI) under visible light irradiation. Compared with previous reports,2,3,7,9 the fascinating photo-catalytic performances have been demonstrated by using the minimum amount of catalyst for the fastest completely reduction of the highest concentration of K2Cr2O7 solution.
The fabrication of Sn2S3 nano-materials by using pulsed laser ablation of bulk Sn target in liquid (see ESI† for the synthetic details) is similar to that described in previous studies.11–15 After laser fabrication, the products were carefully washed in distilled water, and centrifuged at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min by an ultracentrifuge. Different amounts of Sn2S3 nano-materials and 5 μL HCl were separately added in 50 mL dichromate (K2Cr2O7)-distilled water solution. The photo-catalytic reduction of aqueous Cr(IV) were carried out at visible-light (15 W, ∼50 lm W−1, 400–750 nm) irradiation in the reaction vessel.
000 rpm for 20 min by an ultracentrifuge. Different amounts of Sn2S3 nano-materials and 5 μL HCl were separately added in 50 mL dichromate (K2Cr2O7)-distilled water solution. The photo-catalytic reduction of aqueous Cr(IV) were carried out at visible-light (15 W, ∼50 lm W−1, 400–750 nm) irradiation in the reaction vessel.
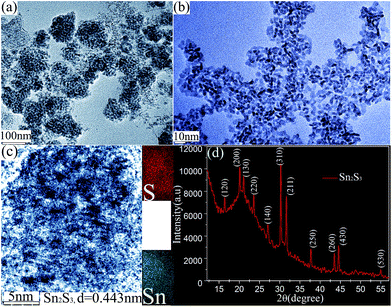
Before the photo-catalytic reduction, the detailed structures of Sn2S3 nano-materials were firstly analyzed in Fig. 1. The transmission electron microscopy (TEM) image of nano-materials in Fig. 1(a) obtained by using 5 μL HCl in solution during laser ablation. It shows that numerous quasi-spherical nano-materials with the diameter of ∼100 nm are porous structures with obvious surface pores, and accreted with each other (see also Fig. S1, ESI†). The pores in nano-structures (Fig. 1(a)) are shown as contrasting light images with their walls as darker ones and porous as brighter ones due to different penetration depths of the incident electron beam. Increasing the amount of HCl to 10 μL in solution, we found that the ultra-small nano-particles with size of sub-10 nm can be fabricated after laser ablation of Sn in TAA liquid (Fig. 1(b)). It is noticeably smaller than that in Fig. 1(a). In the larger region, the numerous liquid-dispersed sub-10 nm nano-particles are fabricated one by one separately, and almost not hinge jointed (Fig. S2, ESI†). The high resolution TEM image in Fig. 1(c) provides the structural detail of the representative ultra-small porous nano-particles. It is found to be well crystalline according the clear lattice fringes. Correspondingly, the lattice fringes with spacing of 0.443 nm can be indentified for Sn2S3 (200) plane. In addition, elemental mapping image of the typical nano-particle in Fig. 1(c) also clearly demonstrates that the well dispersed Sn and S elements are homogeneously present, and the relative ratio of Sn to S is measured about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Moreover, the crystallographic investigation of the ultra-small nano-structures was established by X-ray diffraction (XRD) in Fig. 1(d). Based on the Scherrer equation, the crystallite size is approximately 9 nm, which is consistent with the particle size observed by TEM image. The XRD pattern clearly reveals that a series of (120), (200), (130), (220), (140), (310), (211), (250), (260), (430) and (530) Sn2S3 diffraction peaks centred at 16.102°, 20.027°, 21.498°, 23.771°, 27.334°, 30.916°, 31.936°, 37.933°, 43.692°, 45.305° and 55.403° (JCPDS no. 14-0619) were indeed detected. The above mentioned results clearly confirm that the single crystal Sn2S3 porous nano-particles with sub-10 nm size can be fabricated by laser ablation of Sn in activated solution. At the moment of pulsed laser arriving at Sn target, rapid boiling and vaporization of Sn element will occur, resulting the formation of explosive Sn plasma with ultra-high temperature (∼thousands celsius) on the irradiated spot.14–16 The hot plasma in the solution should significantly improve the surrounded TAA hydrolyzing degrees. The nucleation of Sn and S (from TAA hydrolyzing reactions) will take place in the stage of rapid condensation of the plasma, and sharply terminate due to expiration of the pulse and exhaustive expansion of the Sn vapor (a few microseconds). The HCl in activated solution plays a critical role in the formation of porous structure. It can enable some Sn and S elements to be dissolved and removed from the hybrid nano-composites owing to the ultra-rapid acid etching in the early stage of the nucleation process. The higher degree of acid etching is strongly related to the amount of HCl in the solution, resulting in the formation of ultra-small porous nano-particles.
3. Moreover, the crystallographic investigation of the ultra-small nano-structures was established by X-ray diffraction (XRD) in Fig. 1(d). Based on the Scherrer equation, the crystallite size is approximately 9 nm, which is consistent with the particle size observed by TEM image. The XRD pattern clearly reveals that a series of (120), (200), (130), (220), (140), (310), (211), (250), (260), (430) and (530) Sn2S3 diffraction peaks centred at 16.102°, 20.027°, 21.498°, 23.771°, 27.334°, 30.916°, 31.936°, 37.933°, 43.692°, 45.305° and 55.403° (JCPDS no. 14-0619) were indeed detected. The above mentioned results clearly confirm that the single crystal Sn2S3 porous nano-particles with sub-10 nm size can be fabricated by laser ablation of Sn in activated solution. At the moment of pulsed laser arriving at Sn target, rapid boiling and vaporization of Sn element will occur, resulting the formation of explosive Sn plasma with ultra-high temperature (∼thousands celsius) on the irradiated spot.14–16 The hot plasma in the solution should significantly improve the surrounded TAA hydrolyzing degrees. The nucleation of Sn and S (from TAA hydrolyzing reactions) will take place in the stage of rapid condensation of the plasma, and sharply terminate due to expiration of the pulse and exhaustive expansion of the Sn vapor (a few microseconds). The HCl in activated solution plays a critical role in the formation of porous structure. It can enable some Sn and S elements to be dissolved and removed from the hybrid nano-composites owing to the ultra-rapid acid etching in the early stage of the nucleation process. The higher degree of acid etching is strongly related to the amount of HCl in the solution, resulting in the formation of ultra-small porous nano-particles.
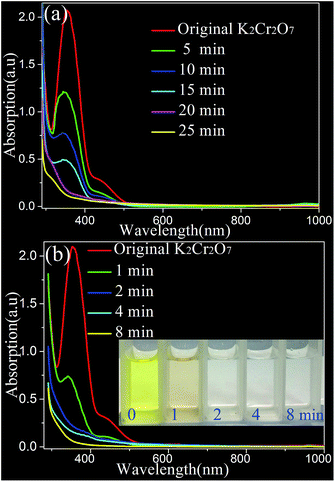
Fig. 2(a) and (b) show the photocatalytic reductions of 50 mL of 1 × 10−3 M Cr(VI) aqueous solution under visible light irradiation in the presence of the as-synthesized large sized (∼100 nm) and sub-10 nm Sn2S3 porous nano-particles, respectively. The dosage of the photo-catalyst in each dichromate solution is 10 mg. As shown in Fig. 2(a), the main absorption band of Cr(VI) centred at about 362 nm drastically decreases with the exposure time. It is observed that the as-prepared Sn2S3 porous nano-particles with diameter of about 100 nm inherit good photo-catalytic reducing capability. The Cr2O72− ions are completely reduced within 25 min of visible light irradiation. On the other hand, the HCl in the solution plays an important role in the photo-catalytic reduction of Cr(VI). Without hydrogen chlorides, the main absorption of Cr(VI) will not reduce anymore after 5 min visible light irradiation (see also Fig. S3, ESI†). The inevitable deposition of Cr(OH)3 on the surface of the catalysts will significantly reduce their photo-catalytic efficiency and activity, which can be easily removed by acid in the solution. Interestingly, some more fascinating improved photo-catalytic activity by using ultra-small Sn2S3 porous nano-particles, as shown in Fig. 2(b). The absorption peak of Cr(VI) sharply dropped from 2.1 to 0.004 a.u (the reduction of nearly 99.8% Cr(VI)) with exposure time of 8 min. This is also agreement with the gradual colour change of the solution from yellow to colourless with irradiation time prolonging from 0 to 8 min. The reduction time is about one third of the result by using large sized Sn2S3 nano-particles. Compared with the reduction in Fig. 2(a), the enhanced photo-catalytic activity of the sub-10 nm Sn2S3 nano-particles should be highly related to the ultra-small porous-structure. It is well known that the visible-light derived photo-catalytic reduction of Cr(VI) is entirely affected by photo-excited electron and facilitated by well-dispersed electron–hole structure.3 The dispersed electron–hole is easily to be formed in the ultra-small Sn2S3 porous structures with higher surface area than that large ones, and relative narrow energy band gap (about 2.05 eV calculated from Fig. S4†).
For a photo-catalyst to be useful, it should be stable under repeated application. To test the stability and reusability of Cr(VI) photo-catalytic-reduction by the ultra-small Sn2S3 porous nano-particles, we further carried out the experiments repeatedly ten times. After each photo-catalytic-reduction, the catalysts were separated from the aqueous suspension by centrifugation and carefully rinsing in distilled water, dried under vacuum for further use. Fig. 3(a) shows the reduction-time dependence of the relative concentration C/C0 of the Cr(VI) and the recycling test of photo-catalytic-reduction of Cr(VI) by using 10 mg ultra-small Sn2S3 nano-cages. Where C and C0 are the concentration of Cr(VI) after visible light irradiation and initial solution, respectively. The photo-catalyst of Sn2S3 porous nano-particles shows excellent photo-stability under five repeated applications with nearly constant photodecomposition rate. After tenth cycles of photo-catalytic reduction of Cr(VI), there is still 94.2% of Cr(VI) can be reduced with the exposure time of 8 min, and 98.7% of Cr(VI) will be reduced within 12 min exposure time. The TEM image of Sn2S3 nano-particles (the inset in Fig. 3(b)) at the end of the tenth repeated photo-catalytic-reduction is almost identical to that of the as-prepared sample. The slightly reduced photo-catalytic efficiency should be caused by the inevitable deposited Cr(III) species in the pore of nano-particles and then pore blockage, which cannot be completely dissolved by HCl in the solution after tenth repeated experiments. Meanwhile, the activity decrease in the reusability tests might also be due to the loss of materials during isolation after each run. Finally, the curve of the required catalyst dosages for completely reduction of Cr(VI) as a function Cr(VI) concentration in 300 mL solution is displayed in Fig. 3(b). It will offer some valuable quantitative information of photo-catalytic reduction of different amount aqueous Cr(VI), which is great signification for the specific applications. The exposure time for completely reduction of Cr(VI) was located at 8 min in each experiment. As shown in Fig. 3(b), the dosage of catalyst almost linearly increases with the concentration of Cr(VI) in region of 0–150 mg L−1. Then, the required dosage of catalyst exponential increases with the higher concentration of Cr(VI). It is reasonable to deduced that the dense Cr(VI) in liquid (>150 mg L−1) requires much more Sn2S3 nano-cages for photochemical reduction.
The reason can be partly explained by the recombination and agglomeration of catalyst at higher concentration (see TEM image of 120 mg/300 mL Sn2S3 in Fig. S5†). Taking advantage of the quantitative results in Fig. 3(b), the comparisons of photo-catalytic reduction of Cr(VI) between ultra-small Sn2S3 porous nano-particles and previously reports using SnS2, SnS2/SnO2 and SnS2/TiO2 are shown in this paper (Table 1). Compared with previous reports, the ultra-small Sn2S3 porous nano-particle fabricated in this paper is an excellent photo-catalyst with enhanced activity and high stability in Cr(VI) reduction under visible light irradiation. The designed ultra-small Sn2S3 porous nano-particles with the fascinating photo-catalytic performances have significant implications for polluted waters treatment in the further.
| Photo-catalytic material | Amount of catalyst | Amount of K2Cr2O7 | Required time | Ref. |
|---|---|---|---|---|
| SnS2 | 50 mg | 2 × 10−4 M, 50 mL | 90 min | 3 |
| Our work (Sn2S3) | 10 mg | 1 × 10−3 M, 50 mL | 8 min | |
| SnS2/SnO2 | 300 mg | 500 mg L−1, 300 mL | 40 min | 7 |
| 20 mg | 10 mg L−1, 20 mL | 30 min | 9 | |
| Our work (Sn2S3) | 9 mg | 50 mg L−1, 300 mL | 8 min | |
| SnS2/TiO2 | 40 mg | 100 mg L−1, 80 mL | 80 min | 2 |
| Our work (Sn2S3) | 22 mg | 100 mg L−1, 300 mL | 8 min |
In conclusion, we have demonstrated the successful synthesize of porous Sn2S3 nano-particles with a mean size of sub-10 nm by pulsed laser ablation of Sn target in activated liquid. The HCl in the TAA solution plays an important role in the formation of ultra-small porous-like structure, due to the rapid acid etching then dissolved of Sn and S ion in the early stage of the nucleation process. Benefiting from the unique structural features, the as-prepared ultra-small Sn2S3 porous nano-particles exhibit excellent photo-catalytic activity and stability in reduction of aqueous Cr(VI) under visible-light irradiation. The simplistic, single-step and versatile strategy developed in this work does not require seed preparations and any intermediate workup process. The work will offer a convenient and valuable way to fabricate highly efficient and stable photo catalysts, and then inspire deeper investigations for photo-catalytic-reduction of other toxic elements from the environment.
Acknowledgements
This work was supported by the Natural Science Foundation of China under Grant No. 11575102, 11105085, 11275116 and 11375108, the Fundamental Research Funds of Shandong University under Grant No. 2015JC007.Notes and references
- W. Wang, M. O. Tade and Z. P. Shao, Chem. Soc. Rev., 2015, 44, 5371–5408 RSC.
- J. G. Wang, X. R. Li, X. Li, J. Zhu and H. X. Li, Nanoscale, 2013, 5, 1876–1881 RSC.
- C. Mondal, M. Ganguly, J. Pal, A. Roy, J. Jana and T. Pal, Langmuir, 2014, 30, 4157–4164 CrossRef CAS PubMed.
- D. K. Padhi and K. Parida, J. Mater. Chem. A, 2014, 2, 10300–10312 CAS.
- K. Maeda, T. Ohno and K. Domen, Chem. Sci., 2011, 2, 1362–1368 RSC.
- I. Kim, H. A. Hosein, D. R. Strongin and T. Douglas, Chem. Mater., 2002, 14, 4874–4879 CrossRef CAS.
- Y. C. Zhang, L. Yao, G. Zhang, D. D. Dionysiou, J. Li and X. Du, Appl. Catal., B, 2014, 144, 730–738 CrossRef CAS.
- R. Wu, Y. Xu, R. Xu, Y. Huang and B. Zhang, J. Mater. Chem. A, 2015, 3, 1930–1934 CAS.
- L. Mao, J. Li, Y. Xie, Y. Zhong and Y. Hu, RSC Adv., 2014, 4, 29698–29701 RSC.
- A. K. Shwarsctein, Y. S. Hu, A. J. Forman, G. D. Stucky and E. W. Mcfarland, J. Phys. Chem. C, 2008, 112(40), 15900–15907 Search PubMed.
- S. Li, M. Chen and X. D. Liu, Opt. Express, 2014, 22(15), 18707–18714 CrossRef PubMed.
- S. Li, D. M. Wang, Z. Y. Wang, Z. W. Wang, M. Chen and X. D. Liu, RSC Adv., 2015, 5, 63233–63239 RSC.
- Z. J. Yan, R. Q. Bao and D. B. Chrisey, Langmuir, 2011, 27(2), 851–855 CrossRef CAS PubMed.
- H. B. Zeng, X. W. Du, S. C. Singh, S. A. Kulinich, S. K. Yang, J. P. He and W. P. Cai, Adv. Funct. Mater., 2012, 22, 1333–1353 CrossRef CAS.
- G. W. Yang, Prog. Mater. Sci., 2007, 52, 648–698 CrossRef CAS.
- V. Amendola and M. Meneghetti, Phys. Chem. Chem. Phys., 2013, 15, 3027–3046 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental, the low magnification TEM images of the nano-materials, photocatalytic reduction of Cr(VI) without any HCl in solution, UV-visible absorption of ultra-small nano-materials, and TEM images of catalyst at high concentration. See DOI: 10.1039/c6ra00244g |
| This journal is © The Royal Society of Chemistry 2016 |