Supermolecular assembly of polyoxoanion and metal–organic cationic units towards a model for core–shell nanostructures†
Abstract
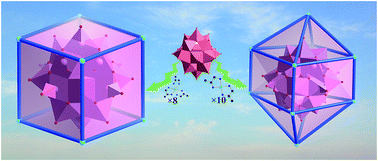
Two novel core–shell-like molecular composites, [Ni(dap)3]4[HVIV12VV6O42(PO4)] (1) and [Ni(dap)2]4[HVIV12VV6O42(PO4)]·4H2O (2) (dap = 1,2-diaminopropane), were obtained from the supermolecular assembly of polyoxoanions and metal–organic cationic units. These two inorganic–organic hybrids were prepared under hydrothermal conditions and characterized by elemental analysis, IR spectroscopy, PXRD and single crystal X-ray diffraction analysis. In the well defined molecular core–shell structure of composite 1, the sphere-like anion [PV18O46]9− was surrounded by a shell composed of eight windmill-type [Ni(dap)3]2+ cationic units. In 2, ten sheet-like [Ni(dap)2]2+ metal–organic groups surrounded the sphere-like anion [PV18O46]9−, which was achieved by the easy alteration of the chemical composition of the core–shell structure by simply adjusting the feeding amount of dap. The resulting core–shell-like molecular composites represent a promising structural model toward core–shell nanostructures and exhibit electrocatalytic activity for the reduction of H2O2 and oxidation of NO2−.

 Please wait while we load your content...
Please wait while we load your content...