Porous silicon nano-aggregate from silica fume as an anode for high-energy lithium-ion batteries†
Abstract
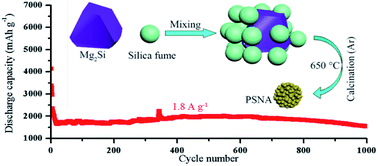
A porous silicon nano-aggregate (PSNA) has been prepared using a ‘‘conproportionation’’ reaction of magnesium silicide and silica fume, which is a waste by-product from the manufacture of metallurgical grade silicon. The as-prepared PSNA, with a pore diameter of 10–100 nm, is composed of Si nanoparticles with diameters of 10–50 nm. As an anode for a rechargeable lithium ion battery, it delivers a high reversible specific capacity (3224 mA h g−1 at 0.36 A g−1) and significant cycling stability (~90% capacity retention after 500 cycles and ~69% capacity retention even after 1000 cycles at a current density of 1.8 A g−1).

 Please wait while we load your content...
Please wait while we load your content...