Nanotechnology to rescue bacterial bidirectional extracellular electron transfer in bioelectrochemical systems
Abstract
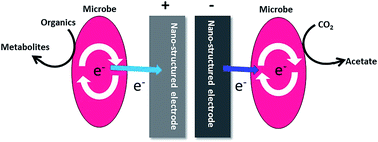
An electrically active bacterium transports its metabolically generated electrons to insoluble substrates such as electrodes via a process known as extracellular electron transport (EET). Bacterial EET is a crucial process in the geochemical cycling of metals, bioremediation and bioenergy devices such as microbial fuel cells (MFCs). Recently, it has been found that electroactive bacteria can reverse their respiratory pathways by accepting electrons from a negatively poised electrode to produce high-value chemicals such as ethanol in a process termed as microbial electrosynthesis (MES). A poor electrical connection between bacteria and the electrode hinders the EET and MES processes significantly. Also, the bidirectional EET process is sluggish and needs to be improved drastically to extend its practical applications. Several attempts have been undertaken to improve the bidirectional EET by employing various advanced nanostructured materials such as carbon nanotubes and graphene. This review covers the recent progress in the bacterial bidirectional EET processes using advanced nanostructures in the light of current understandings of bacteria–nanomaterial interactions.

 Please wait while we load your content...
Please wait while we load your content...