The spin-dependent transport of transition metal encapsulated B40 fullerene
Abstract
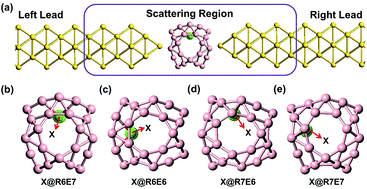
The all-boron fullerene, B40, has been successfully experimentally synthesized [Zhai et al., Nat. Chem., 6, 727 (2014)]. Compared with C60, the smaller cage-like structure is more suitable to dope with metal atoms. Based on density functional theory and nonequilibrium Green’s function method, we investigate the spin-dependent transport of transition metal atom-encapsulated B40 fullerene, i.e., X@B40 (X = Fe, Mn, Ni, and Co), which are contacted with Au electrodes. The transmission spectra of Fe- and Mn-doped systems are spin-polarized, and those of Ni-doped ones are spin-unpolarized. Interestingly, in Co-doped systems, the transmission is highly spin-polarized for the hexagonal doping case, but spin-unpolarized for the heptagonal doping case. Further investigation shows that the screening effect of the electrodes on the magnetism of Co is the underlying physical mechanism, which is found to be robust to the electrode material. We believe that these findings are very useful for developing spintronic devices.

 Please wait while we load your content...
Please wait while we load your content...