Enhanced piezoelectric properties and constricted hysteresis behaviour in PZT ceramics induced by Li+–Al3+ ionic pairs†
Y. Fenga,
W. L. Liab,
D. Xua,
W. P. Caoa,
Y. Yua and
W. D. Fei*a
aSchool of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: wdfei@hit.edu.cn
bNational Key Laboratory of Science and Technology on Precision Heat Processing of Metals, Harbin Institute of Technology, Harbin 150001, P. R. China
First published on 5th April 2016
Abstract
Recently, we proposed a new approach (Li+–Al3+ ionic pairs doping) to enhance the piezoelectric properties of BaTiO3 ceramic. Here we sequentially prove that Li+ and Al3+ can also enhance the piezoelectric properties of PbZr0.52Ti0.48O3 (PZT) ceramic. The X-ray diffraction results demonstrate the optimized distribution of Li+–Al3+ ionic pairs parallel to the [001] direction in the doped ceramics, as same as the results in BaTiO3-based ceramic. The doped ceramics exhibit constricted hysteresis loops, which is associated with the restoring force of Li+–Al3+ ionic pairs as a result of the Coulomb force between Li+ and Al3+ ions. The results show that the piezoelectric properties and corresponding temperature stability of the doped ceramics were enhanced significantly. The analysis and conclusion indicate that the piezoelectric mechanism coming from ionic pairs applies equally well to the PZT ceramic. Co-doping Li+ and Al3+ into the A-site is a preferable approach for the piezoelectric property enhancement of ABO3 ferroelectric ceramics.
Introduction
Piezoelectric materials are used in sensor and actuator technologies because of their ability to couple electrical and mechanical displacement, resulting in electrical polarization changes in response to applied mechanical stress and/or strain that can generate an electric field.1,2 Actuator applications require a high piezoelectric constant and electromechanical coupling providing high strain with high force, for example ultrasonic motors.1,2 It is well known that phase instabilities are responsible for optimizing the piezoelectric and dielectric properties of ferroelectric ceramics, and have thus been of great interest to the ferroelectric community for more than half a century. Usually, there are two kinds of methods to obtain high piezoelectric properties in the ABO3-type piezoelectric ceramic.One is to design a morphotropic phase boundary (MPB) in the ABO3 systems, as in the case for PbZrO3–PbTiO3 (PZT).3 It is well known that Jaffe et al. introduced the concept of MPB from the PZT system in an unlimited range solid solution of PbTiO3 and PbZrO3.3 The enhancement of the piezoelectric properties in PZT occurs near the boundary of the composition–temperature phase diagram where crystal structure changes from tetragonal (T) to rhombohedral (R).4 This boundary is known as the MPB which is nearly vertical along the temperature scale.5 According to the large piezoelectric mechanism found in the PZT, researchers began to investigate the possibility of MPB in the lead-free piezoelectric ceramic by doping various ABO3-type perovskite, such as (Bi1/2Na1/2)TiO3–BaTiO3 and Ba(Zr0.2Ti0.8)O3–(Ba0.7Ca0.3)TiO3 systems. (Bi1/2Na1/2)TiO3–BaTiO3 (BNT–BT) system has been of great interest since the discovery of an MPB, where a rhombohedral and a tetragonal symmetry coexist.6–8 The interest has arisen largely because the MPB was reported to separate the rhombohedral BNT and the tetragonal BT as in PZT, accompanied by a significant enhancement in the dielectric permittivity and electromechanical coupling factor as well as piezoelectric properties. Another important lead-free piezoelectric system that includes MPB was found and developed in Ba(Zr0.2Ti0.8)O3–(Ba0.7Ca0.3)TiO3 (BZT–BCT) system, where high piezoelectricity starts from a point of a paraelectric cubic phase (C), ferroelectric rhombohedral (R), and tetragonal (T) phases.9–11 This point was further proved to be a tricritical point by the vanishing thermal hysteresis and highest transition permittivity peak at the point. In the vicinity of triple critical point, the system has a flattened energy landscape and consequently a low energy barrier for polarization rotation. In terms of previous studies, several essential characteristics which a MPB should possess can be recapitulated as follows. Firstly, two different end members with almost the same type of structure could form an unlimited solid solution and in the MPB region it has the characteristic of compositional homogeneity.4,6 Secondly, although a MPB was initially defined as a boundary with equal amounts of two separated phases with similar structure, in fact, the exact composition location of the MPB was hardly determined. Therefore, in fact, the MPB represents a narrow composition region with the coexistence of two different phases or a transitional phase with lower symmetry.5,12,13
Another method to obtain high piezoelectric properties is to design polymorphic phase transition (PPT) in a composition system. A typical PPT was found in K0.5Na0.5NbO3 (KNN) solid solution.14–17 The enhanced properties of the KNN-based piezoelectric ceramics are related to the process of PPT, which also known as the orthorhombic-tetragonal ferroelectric phase transition. By shifting the phase transition temperature TO–T downward, it leads to the coexistence of the orthorhombic phase and the tetragonal phase at room temperature, and exhibits the analogous effect of a morphotropic phase boundary in the PZT system.18 Considering previous studies, several essential characteristics which a PPT should possess can be recapitulated as follows. On the one hand, two ferroelectric phases should coexist at room temperature, which is beneficial to polarization rotation and piezoelectric effect. On the other hand, the high piezoelectric properties induced by PPT are very sensitive to temperature since the coexistence stage of two ferroelectric phases will disappear with the temperature increasing.17,19
Recently, an easy and novel method (neither the MPB nor the PPT) is proposed to achieve high piezoelectric properties in the ABO3 ferroelectrics.20–22 Our recent work indicates that the replacement of Ba2+ by the smaller Li+ and Al3+ ions in the A-sites can form Li+–Al3+ ionic pairs and greatly improve the piezoelectric properties of BaTiO3-based ceramics.20 The reason why we choose the Li+ and Al3+ is discussed in ESI.† XRD results proved precisely the optimized distribution of Li+–Al3+ ionic pairs parallel to the [001] direction in the lattice. After the comprehensive analysis, we proposed a new approach to obtain large piezoelectric effect, which is associated with appropriate ionic pairs doping. In this study, similar research process has been applied for the PZT system. The XRD patterns demonstrate the same distribution of Li+–Al3+ ionic pairs compared with BaTiO3 ceramic doped by Li+–Al3+ ionic pairs. Above all, we discovered a kind of dipole polarization existing in the doped PZT ceramic, which resulted in constricted hysteresis loops. Through comparing the hysteresis loops measured after different thermal treatment, the effect of dipole polarization (PD) caused by Li+–Al3+ ionic pairs on the spontaneous polarization was researched. For the piezoelectric properties, the results finally confirm that doping Li+–Al3+ ionic pairs can enhance the piezoelectric constant and electromechanical coupling factor, as well as the thermal stability of piezoelectric properties. In a word, the enhanced effect of Li+–Al3+ ionic pairs on the piezoelectric properties in PZT-based ceramic is identical to that in BaTiO3-based ceramic. The results and analysis in the PZT-based ceramics justify the validity of Li+–Al3+ ionic pairs again. Compared with MPB, researcher can easily take advantage of ionic pairs doping to obtain large piezoelectric properties in ceramics instead of finding narrow MPB region. In addition, the temperature stability testing reveals that the depolarization temperature of these doped ceramics are greatly improved, even close to their Curie temperatures, which can't be acquired by designing PPT. The mechanism (Li+–Al3+ ionic pairs) will provide a new method to achieve large and stable piezoelectric response in a wide range of ABO3-type perovskite systems.
Experimental
Materials and methods
Pb1−2xLixAlxZr0.52Ti0.48O3 ceramics with Li+ and Al3+ contents of 0, 0.5, 1, 1.5, and 2 mol% were prepared by conventional ceramic processing techniques using the following reagent-grade raw materials: Pb2O3 (99.9%), TiO2 (99.9%), ZrO2 (99.9%), Li2O (99.9%) and Al2O3 (99.9%). The doped ceramics including Li+ and Al3+ will be called PLAZT for short in present study. All chemicals were purchased from Aladdin Industrial Corporation. An excess of 3 mol% Pb2O3 was included to compensate for Pb loss during sintering. The starting reagents were ball milled in ethanol for 15 h. After ball-milling, the resulting slurries were dried at 80 °C for 12 h, then calcined in an alumina crucible at 800 °C for 2 h. The calcined powders were then ball milled in ethanol for 15 h using ZrO2 ball-milling media, followed by drying the slurries at 80 °C for 12 h. The dried powders were pressed into pellets of 10 mm in diameter and 1 mm in thickness using a few drops of 5 wt% polyvinyl alcohol (PVA) as a binder. After burning off the PVA, the PLAZT pellets embedded in remaining powders were sintered at 1200 °C for 2 h at a rate of 5°C min−1 in covered alumina crucibles.Characterization
The crystalline structure of each sample were collected using a Philips X'Pert diffractometer with Cu Kα radiation, operating at 40 kV and 40 mA, in the 2θ ranges of 20–80° with a step size of 0.05°and 0.5 s per step. The fine scan XRD patterns were carried out with a step size of 0.02°and 5 s per step. The morphology and grain size were examined on a FEI Nanolab 600i scanning electron microscopy (SEM). Platinum electrodes were evaporated onto the ceramic surfaces and then annealed at 500 °C for 30 min to allow for electrical properties characterization.The polarization vs. electric field (P–E) hysteresis loops were determined using a Radiant Technologies Precision work station. There are two groups of loops are carried out after different treatment: thermal annealing and thermal quenching. Thermal annealing experiments were heating the samples to 500 °C (above the Curie temperature of PZT ceramics) for 30 min, followed by a subsequent furnace cooling to room temperature. Thermal quenching experiments were performed by holding the samples at 500 °C for 30 min, and subsequent air cooling on a cold metallic plate to room temperature.
For the piezoelectric properties, samples were poled at 120 °C in a silicone oil bath under a DC field of 3 kV mm−1 for 30 min. The piezoelectric constant (d33) was measured employing a quasi-static piezoelectric constant testing meter (ZJ-4AN, Institute of Acoustics, Chinese Academy of Science). The electromechanical coupling factor (kp) of every poled samples were calculated via a resonance–antiresonance method using an Agilent 4294A impedance analyzer. The thermal stability of d33 was measured via separate ex situ thermal depoling measurements. The measurements were completed in which the samples were annealed at a set temperature for 30 min in a furnace, after which they were removed and the d33 was measured when the sample reached room temperature. Each reported reading was an average of several readings at different positions on the face of the pellet. The set temperature was progressively increased until the samples exhibited a near-zero d33 value.
Results and discussion
Phase and microstructure characterization
Fig. 1(a) shows the XRD patterns of PZT and PLAZT ceramic powders with different Li+ and Al3+ contents in the 2θ range of 20–80° measured at room temperature. All diffraction peaks match well with the ABO3-type perovskite phases. The diffraction data confirms that both PZT and PLAZT powders exhibit tetragonal symmetry (PDF no. 33-0784), as evidenced by the relative intensity ratios of the 002/200 and the existence of a single 111 peak at 2θ = 38.9°.In order to obtain the location of Li+ and Al3+ ions in the PLAZT lattice, fine scan XRD patterns measured at room temperature in the range of 27.5–28.5° and 43–45.5°, corresponding to the 111 and 200 peaks were shown in Fig. 1(b) and (c), respectively. In the Fig. 1(b), the right shift of 111 peak is observed with Li+ and Al3+ content increasing, which indicates that both ions are incorporated into lattice because the radius of Li+ and Al3+ is far smaller than radius of Pb2+. After the introduction of Li+ and Al3+, however, a distinct change in the relative intensity ratio of 002/200 peaks (here after referred to as I002/I200) was observed for the PLAZT ceramic powders in the Fig. 1(c). Fig. 1(d) shows that the (I002/I200)x/(I002/I200)0 value (where the superscripts 0 and x denote PZT and PLAZT ceramic powders, respectively) tends to decrease firstly, and then increase with Li+ and Al3+ content increasing. This result is attributed to the preferential distribution of Li+–Al3+ ionic pairs parallel to c axis ([001] direction) as explained in our previous papers and ESI.† 20,23 Fig. 1(e) presents a diagram of the lattice distortion induced by the preferential distribution of Li+–Al3+ ionic pairs parallel to the [001] direction. As Li+–Al3+ content increasing (0–1 mol%), the lattice distortion induced by Li+–Al3+ ionic pairs parallel to the [001] direction mainly impact the diffraction intensity of the 002 peak rather than 200 peak, which brings on reduced (I002/I200)x/(I002/I200)0. When the Li+ and Al3+ are further added (≥1 mol%), the number of [100] and [010]-oriented Li+–Al3+ ionic pairs begins to increase as a result of elastic energy limitations, resulting in the uptrend of the (I002/I200)x/(I002/I200)0.
Fig. 2 exhibits the scanning electron micrographs of the Pb1−2xLixAlxZr0.52Ti0.48O3 (where x = 0, 0.5, 1.5 and 2 mol%, respectively) ceramics. In general, the PZT and PLAZT ceramics show dense and homogeneous microstructures. The effect of the Li+ and Al3+ on the microstructure of the ceramics is obvious. Inhomogeneous and irregular shape grains whose size is in the range of 10–20 μm are found on the surface of PZT ceramic, as shown in Fig. 2(a).
 | ||
| Fig. 2 Surface SEM micrographs of PLAZT ceramics: (a) x = 0 mol%, (b) x = 0.5 mol%, (c) x = 1.5 mol% and (d) x = 2 mol%. | ||
However, the grain of ceramics doped by 0.5, 1.5 and 2 mol% Li+ and Al3+ (Fig. 2(b)–(d)) are comparatively uniform, whose size in the range of 2–10 μm. In addition, the grains in the PLAZT ceramics exhibit obviously sphere shape and smaller size than that of pure PZT ceramic. Evidently, the Li+ and Al3+ doping plays a role in grain refinement.
Constricted hysteresis behaviour
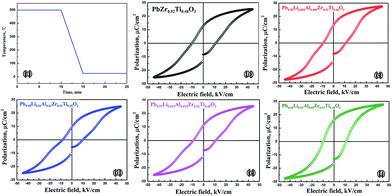
Lowered temperature curve during the annealing (cooling down from 500 °C to room temperature slowly) is shown in Fig. 3(a). The P–E hysteresis loops for the ceramics at room temperature measured after thermal annealing are shown in Fig. 3(b)–(f). PZT ceramic shows a typical ferroelectric behaviour that is distinct in terms of both significant saturated polarization (2PS = 41.7 μC cm−2) and remnant polarization (2Pr = 25.6 μC cm−2). There are constricted hysteresis loop observed in Fig. 3(c)–(e), corresponding to the PLAZT ceramics doped by 0.5, 1 and 1.5 mol%, respectively. The constricted hysteresis loop should not be attributed to antiferroelectric phase of PbZrO3 because the ratio of Zr/Ti is only 52/48 rather than larger value. Hence, the constricted behaviour in hysteresis loops gradually come out in the Li+–Al3+ content range of 0–1 mol%, and then slowly disappear when Li+–Al3+ content is further added. Interestingly, the most obvious constricted hysteresis loop is discovered in the Pb0.98Li0.01Al0.01Zr0.52Ti0.48O3 (1 mol%) ceramic, in which I002/I200 is relatively low compared with other ceramics. Through a comparative analysis on the hysteresis loops between PZT and Pb0.98Li0.01Al0.01Zr0.52Ti0.48O3, we found that the saturated polarization in both ceramics is almost identical, but remnant polarization of Pb0.98Li0.01Al0.01Zr0.52Ti0.48O3 is smaller than that of PZT. The analysis above indicates that when the electrical field is applied on the two sides of both ceramics, the domain switching behaviours in above both ceramics are almost identical. The difference is that a part of domains possibly return to original state in the Pb0.98Li0.01Al0.01Zr0.52Ti0.48O3 ceramic after the electrical field is removed, resulting in the lower remnant polarization. We thought this constricted behaviour should be attributed to certain restoring force existing in the lattice.24–26 Considering the distribution and effect of Li+–Al3+ ionic pairs, the restoring force is possibly caused by dipole polarization (PD) of Li+–Al3+ ionic pairs accompanied by Coulomb force between Li+ and Al+ ions.To further investigate the formation process of constricted hysteresis loop in the PLAZT ceramics, the P–E hysteresis loop for all the ceramics at room temperature measured after thermal quenching (cooling down from 500 °C to room temperature quickly) are carried out again and P–E loops are shown in Fig. 4. Lowered temperature curve during the quenching is shown in Fig. 4(a). All PLAZT ceramics show typical ferroelectric behaviour as well as PZT ceramic. After above thermal treatment, the constricted hysteresis loops in PLAZT ceramics disappear, which demonstrates that there is no restoring force helping the domains return to original state when the temperature cools down just below Curie temperature.
By contrasting the hysteresis loops measured after various treatments (Fig. 3 and 4), the formation mechanism of constricted hysteresis loops, in what follows, are described in detailed. As shown in Fig. 5, the preferential distribution of Li+–Al3+ ionic pairs along the [001] direction possibly comes from the emergence of spontaneous polarization (PS) after C–T phase transition. Above the phase transition point TC, the crystal of PLAZT ceramic is in the paraelectric state and has a cubic symmetry. When the temperature is just below TC, the crystal of PLAZT ceramic changes to ferroelectric state with a spontaneous polarization PS along the [001] direction. In particular, the paraelectric–ferroelectric phase transition of PZT ceramics with ABO3-type perovskite structure belongs to a first order transition, which includes two processes-nucleation and growth of tetragonal ferroelectric phase. The c-axis orientation of tetragonal ferroelectric phase is determined by the nucleus orientation. The nucleation process can be affected by the local electric field, and it is actively favorable for the spontaneous polarization (PS) parallel to electric field. The Li+–Al3+ pair can create an electric dipole moment PD as mentioned above, the local electric fields (ED) formed by the dipole at the head and the tail of the pairs are nearly parallel to PD, and the ED in the middle and at the sides around the dipole are nearly antiparallel to PD (see Fig. 5(a)). The former ED can lead to ferroelectric phase nucleus with PS nearly parallel to PD and the latter ED can induce ferroelectric phase nucleus with PS nearly antiparallel to PD. Therefore, the nucleation of tetragonal phase in the vicinity of Li+–Al3+ dipole causes the coexistence of PS nearly parallel (PS∥PD) and nearly antiparallel to PD(−PS∥PD) in the PZT ceramics (see Fig. 5(b)).20,22
Through the thermal quenching (cooling down from 500 °C to room temperature quickly) as shown in Fig. 5(c), it is too rapid for the tetragonal ferroelectric phase to grow because of short time. At this moment, most of PS around ionic pairs remains the original state similar to the state in Fig. 5(b).
Through the thermal annealing (cooling down from 500 °C to room temperature slowly) as shown in Fig. 5(d), growth processes of tetragonal ferroelectric phase are ongoing owing to the enough time and higher temperature. The PS in region 1 and 3 possess the same direction, but there is opposite direction PS existing in the region 2 (see Fig. 5(b)). Compared with region 1 and 3, the region 2 is obviously small, because the Li+ ion is quite close with Al3+ ion. The area of region 2 in Fig. 5(b) will be easily reduced due to the growth processes of region 1 and 3. Consequently, regions 1, 2 and 3 will integrated into one domain with same direction as shown in Fig. 5(d). The changing of spontaneous polarization in region 2 will finish during the temperature cooling down from TC to room temperature (RT) in the furnace. Finally, there are two kinds of polarizations with same direction coexisting in that domain: one is the spontaneous polarization PS, another is the dipole polarization PD(PD∥PS).
It is noteworthy that there is no constricted hysteresis loops observed in PLAZT ceramic through the thermal quenching (see Fig. 4(d)) and LiAlSiO4/BaTiO3 (BT/LAS) ceramics in our previous study.20 This can be attributed to the following two reasons. For the PLAZT ceramic through the thermal quenching, the nucleation of tetragonal phase in the vicinity of Li+–Al3+ dipole causes the coexistence of PS nearly parallel (PS∥PD) and nearly anti-parallel to PD(−PS∥PD). Consequently, PD is distributed randomly in space and the total net electric field from PD is zero, giving rise to the relatively symmetric. In addition, the Li+ and Al3+ ions locate either near the domain boundary or in the different domains, so the PD can't act as the source of restoring force. For the doped BaTiO3 ceramics, the Curie temperature is far lower (∼120 °C) than that of PZT ceramics. There is no enough time or high temperature for tetragonal phase to grow in the cooling process of 120 °C-RT. In other words, domain state around the ionic pairs at RT is similar to that of PLAZT ceramic after thermal quenching: coexistence of PD∥PS and PD∥−PS; marginalized location of Li+ and Al3+ ions.
For the PLAZT ceramics through the thermal annealing, there are, however, two kinds of polarizations with same direction coexisting in one domain. The type of situation is similar to that in the ceramics including defect dipoles. According to the principle of symmetry conforming defect provided by Ren, the reason of constricted hysteresis loops and detailed discussions are as follows.26–31 If the ionic pair symmetry in each domain follows the tetragonal crystal symmetry, and exhibits a dipole polarization PD aligning along the direction of PS(PD∥PS), as shown in Fig. 6(a), every domain containing PD is in its stable state. The tetragonal crystal symmetry is represented by the large rectangle, while PS is represented by the thick arrow as shown in Fig. 6. The ionic pair symmetry is represented by the small rectangle, while PD is represented by the thin arrow. The stable and unstable states of the domains including ionic pair are also schematically illustrated by the symbol shown in Fig. 6.
When electric field is applied along other direction perpendicular to PS, domain switching occurs abruptly with spontaneous polarization following the field direction (90° domain switching) (see Fig. 6(b)). It is noted that the direction of PD cannot be rotated immediately in such a diffusion less domain-switching process. Once electric field is removed, this ionic pair symmetry and the PD will provide a restoring force or a reverse internal field causing a reverse domain switching (Fig. 6(b) to Fig. 6(c)) because the state of switched domain is unstable (−PD∥PS or PD⊥PS). As a consequence, crystal symmetry will follow the ionic pair symmetry in every domain so that the domain pattern will try best to restore original state. Constricted hysteresis loops in the polarization–electric field (P–E) relation for the PLAZT ceramics (Fig. 6(d)) are obtained, as a result of the restoration of initial multi-domain pattern (which has zero macroscopic/averaged polarization) when the electric field is back to zero. Besides, we notice that the constricted hysteresis loop disappears gradually during the Li+–Al3+ content further increasing (>1 mol%), which is due to the emergence of [100] and [010]-oriented Li+–Al3+ ionic pairs, which is proved at previous XRD analysis. At this moment, parts of PD caused by Li+–Al3+ ionic pairs is vertical to PS(PD⊥PS), thus PD can't deservedly provide the restoring force for 90° domain-switching after removing electric field. Consequently, the constricted hysteresis loops gradually disappears when the Li+ and Al3+ contents are greater than 1 mol% as shown in Fig. 3(d) and (e). The P–E loops of the poled PZT ceramic after prolonged storage measured under different electric field and at different frequencies are shown in Fig. S1 and S2,† respectively. The comparison between ionic pair and defect dipole has been discussed in ESI.†
Effect of ionic pairs on the piezoelectric properties
The variation of piezoelectric constant (d33) and electromechanical coupling factor (kp) with different Li+ and Al3+ contents are shown in Fig. 7(a). It is observed that Li+ and Al3+ substitutions have enhanced the values of piezoelectric constant and electromechanical coupling factor of PZT ceramic significantly. With the Li+ and Al3+ content increasing (0–1 mol%), the d33 and kp increase from 160 to 309 pC N−1 and from 0.36 to 0.51, respectively. As the Li+ and Al3+ content added continuously, both d33 and kp fall. The d33 and kp both reach maximum value at 1 mol%, where the constricted behaviour in hysteresis loops is most obvious and massive Li+–Al3+ ionic pairs is parallel to the [001] direction. It is noticed that the enhanced piezoelectric properties of PLAZT ceramic are caused from doping Li+ and Al3+ ionic pairs in the lattice rather than MPB or PPT. The effect of Li+–Al3+ ionic pairs on the piezoelectric properties in the PZT ceramic is identical to that in the BT ceramic. On the one hand, smaller Li+ and Al3+ can respond immediately to the electrical field, which as a series of dipole contributes to the piezoelectric properties of PLAZT ceramics. On the other hand, the radii of Li+ and Al3+ is far smaller than that of Pb2+, the distortion induced by substitution is larger, which is proved at previous analysis in Fig. 1(c). There are local lattice distortion and lower symmetry than tetragonal symmetry existing in the PLAZT ceramics. It is well known that low symmetry always is connected with high piezoelectric properties.4,32 | ||
| Fig. 7 (a) Piezoelectric constant (d33) and electromechanical coupling factor (kp) (b) temperature dependence of the normalized d33 values of the poled PLAZT ceramics measured ex situ. | ||
Fig. 7(b) shows the temperature dependence of the normalized d33 values of the poled ceramics measured ex situ after annealing the poled samples at various temperatures for 30 min. It is clear that the temperature stability of d33 in PLAZT ceramics is more excellent than PZT ceramic. With the temperature increasing, the d33 of PZT ceramic gradually decreases. However, for the PLAZT ceramics, d33 of all ceramics exhibits high temperature stability until the temperature is close to 370 °C (near their Curie temperature). The phenomenon confirms that the depolarization temperature Td of PLAZT ceramics is quiet high (close to TC), which implies that their stability and reliability are improved. Because the high piezoelectricity in PLAZT ceramics possible originates from the dipole re-orientation of ionic pairs and distortion of lattice induced by Li+–Al3+ ionic pairs, it is too hard to make Li+ and Al3+ return to original site under temperature field.31
Above all, the effect of Li+ and Al3+ on the enhanced piezoelectric properties of PZT ceramic is mainly reflected in two aspects: the enhanced value and thermal stability of d33. The influence of Li+–Al3+ ionic pairs in the PZT ceramic is the same as that in the BT ceramic. We speculate that the approach in the present study is different from any known large piezoelectric mechanism, which includes the PPT and MPB. Our work also indicates that the piezoelectric properties should reach a higher level by doping ionic pairs in ether lead ceramic or lead-free ceramic systems.
Conclusions
Pb1−2xLixAlxZr0.52Ti0.48O3 ceramics with Li+ and Al3+ contents of 0, 0.5, 1, 1.5, and 2 mol% were prepared by conventional ceramic processing techniques. The preferential [001]-distributed Li+–Al3+ ionic pairs were proved in the PLAZT ceramics through XRD patterns analysis. There is obvious restoring force inducing constricted hysteresis loops in the PLAZT ceramics, which is due to the existence of independent dipole polarization. The dipole polarization evidently comes from the Coulomb force between Li+ and Al3+ ions. The formation mechanism of constricted hysteresis loops in the PLAZT ceramics are proposed experimentally and theoretically. Li+–Al3+ ionic pairs in PLAZT ceramics can play a significant role in the enhancement of piezoelectric properties and their thermal stability. This approach and technique (doping ionic pairs) efficiently improves the piezoelectric properties of the ABO3-type perovskite systems, which can be a new guideline for further fabrication of piezoelectric ceramic.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (Grant No. 11272102, and 51471057).Notes and references
- G. H. Haertling, J. Am. Ceram. Soc., 1999, 82, 797–818 CrossRef CAS.
- D. Damjanovic, Rep. Prog. Phys., 1998, 61, 1267–1324 CrossRef CAS.
- B. Jaffe, R. S. Roth and S. Marzullo, J. Appl. Phys., 1954, 25, 809–810 CrossRef CAS.
- D. Damjanovic, Appl. Phys. Lett., 2010, 97, 062906 CrossRef.
- X. H. Du, J. H. Zheng, U. Belegundu and K. Uchino, Appl. Phys. Lett., 1998, 72, 2421–2423 CrossRef CAS.
- W. Jo, J. E. Daniels, J. L. Jones, X. L. Tan, P. A. Thomas, D. Damjanovic and J. Rodel, J. Appl. Phys., 2011, 109, 014110 CrossRef.
- R. Z. Zuo, X. S. Fang, C. Ye and L. T. Li, J. Am. Ceram. Soc., 2007, 90, 2424–2428 CrossRef CAS.
- H. D. Li, C. D. Feng and W. L. Yao, Mater. Lett., 2004, 58, 1194–1198 CrossRef CAS.
- W. F. Liu and X. B. Ren, Phys. Rev. Lett., 2009, 103, 257602 CrossRef PubMed.
- B. Z. Li, J. E. Blendell and K. J. Bowman, J. Am. Ceram. Soc., 2011, 94, 3192–3194 CrossRef CAS.
- D. Z. Xue, Y. M. Zhou, H. X. Bao, J. H. Gao, C. Zhou and X. B. Ren, Appl. Phys. Lett., 2011, 99, 122901 CrossRef.
- J. A. Gallagher, J. Tian and C. S. Lynch, Acta Mater., 2014, 81, 512–523 CrossRef CAS.
- M. Kumar, S. Shankar, O. Parkash and O. P. Thakur, J. Mater. Sci.: Mater. Electron., 2014, 25, 888–896 CrossRef CAS.
- B. Peng, Z. X. Yue and L. T. Li, J. Appl. Phys., 2011, 109, 054107 CrossRef.
- Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya and M. Nakamura, Nature, 2004, 432, 84–87 CrossRef CAS PubMed.
- J. L. Zhang, X. J. Zong, L. Wu, Y. Gao, P. Zheng and S. F. Shao, Appl. Phys. Lett., 2009, 95, 022909 CrossRef.
- R. Z. Zuo, J. A. Fu, D. Y. Lv and Y. Liu, J. Am. Ceram. Soc., 2010, 93, 2783–2787 CrossRef CAS.
- Y. P. Guo, K. Kakimoto and H. Ohsato, Appl. Phys. Lett., 2004, 85, 4121–4123 CrossRef CAS.
- X. P. Wang, J. G. Wu, X. J. Cheng, B. Y. Zhang, D. Q. Xiao, J. G. Zhu, X. J. Wang and X. J. Lou, J. Phys. D: Appl. Phys., 2013, 46, 495305 CrossRef.
- D. Xu, W. L. Li, L. D. Wang, W. Wang, W. P. Cao and W. D. Fei, Acta Mater., 2014, 79, 84–92 CrossRef CAS.
- D. Xu, W. L. Li, L. D. Wang, W. Wang and W. D. Fei, RSC Adv., 2014, 4, 34008–34013 RSC.
- D. Xu, L. D. Wang, W. L. Li, W. Wang, Y. F. Hou, W. P. Cao, Y. Feng and W. D. Fei, Phys. Chem. Chem. Phys., 2014, 16, 13078–13085 RSC.
- Y. M. Wang, X-ray Diffraction of noncrystal and crystal with defects, Science Press, Beijing, 1st edn, 1988, in Chinese Search PubMed.
- X. B. Ren, Nat. Mater., 2004, 3, 91–94 CrossRef CAS PubMed.
- L. X. Zhang and X. Ren, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 174108 CrossRef.
- S. M. Ke, H. T. Huang, H. Q. Fan, H. K. Lee, L. M. Zhou and Y. W. Mai, Appl. Phys. Lett., 2012, 101, 082901 CrossRef.
- L. X. Zhang, W. Chen and X. Ren, Appl. Phys. Lett., 2004, 85, 5658–5660 CrossRef CAS.
- D. M. Lin, K. W. Kwok and H. L. W. Chan, Appl. Phys. Lett., 2007, 90, 232903 CrossRef.
- R. A. Eichel, P. Erhart, P. Traskelin, K. Albe, H. Kungl and M. J. Hoffmann, Phys. Rev. Lett., 2008, 100, 095504 CrossRef PubMed.
- L. X. Zhang and X. Ren, Mater. Sci. Eng., A, 2006, 438, 354–359 CrossRef.
- U. Robels and G. Arlt, J. Appl. Phys., 1993, 73, 3454–3460 CrossRef CAS.
- R. Guo, L. E. Cross, S. E. Park, B. Noheda, D. E. Cox and G. Shirane, Phys. Rev. Lett., 2000, 84, 5423–5426 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00152a |
| This journal is © The Royal Society of Chemistry 2016 |