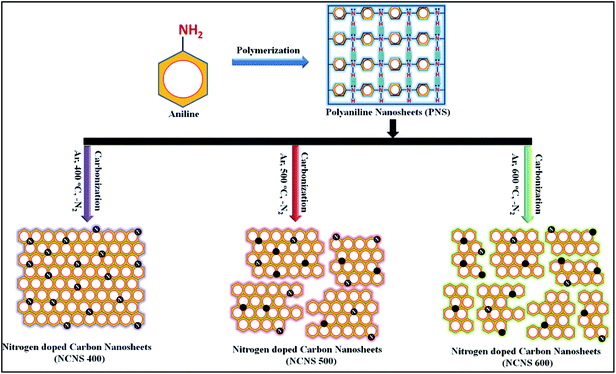
Nitrogen-doped carbon nanosheets for high-performance liquid as well as solid state supercapacitor cells†
Vikrant Sahu,
Sonia Grover,
Gurmeet Singh* and
Raj Kishore Sharma*
Department of Chemistry, Delhi University, Delhi-110007, India. E-mail: drrajksharma@yahoo.co.in; gurmeet123@yahoo.co.in; Tel: +91-11-27666616
First published on 30th March 2016
Abstract
In this article N-doped carbon nanosheets (NCNS), formed by carbonizing polyaniline nanosheets (PNS), are reported. PNS were pyrolyzed at different temperatures (400–600 °C) to get different doping levels of nitrogen in the carbon sheets. The PNS carbonized at 400 °C (NCNS 400) exhibited high electrochemical performance due to a high inter-planar distance of 0.45 nm and high percentage (15 wt%) of N-based functionalities. NCNS 400 shows consistently high charge storage capacity in solid (PVA/H2SO4) and aqueous (1 M H2SO4) electrolytes. The NCNS 400 supercapacitor cell showed high energy density in 1 M H2SO4 (21.6 W h kg−1 at a power density of 293.91 W kg−1) and PVA/H2SO4 (9.8 W h kg−1 at 288 W kg−1). Further, the low relaxation time constant (τo) with low iR-drop and high capacitance retention (99.3%: H2SO4 and 99.8; PVA/H2SO4) is attributed to the improved structural properties upon nitrogen doping in the carbon sheets.
1. Introduction
Due to the depletion of fossil fuel stocks, there is increased attention towards the development of advance energy storage devices. Supercapacitors, as energy storage devices with high cycling life, low cost, fast charge–discharge capacity, high power and energy delivery rates, have found numerous applications in military instruments, home appliances, industrial applications, transportation, portable electronics etc.1–8 Based on the charge storage mechanism these devices are broadly classified in two types: EDLC (electric double-layer capacitor) and pseudocapacitor. Previous is fabricated mainly from carbon allotropes like activated carbons, carbon nanotubes (CNTs), graphene, carbon black, carbon aerogel etc.9,10 while the later utilizes metal oxide and conducting polymers to constitute pseudocapacitance.11,12 Commercially available supercapacitors are mostly fabricated from the activated carbon due to their abundance, low cost, large specific surface area (SSA).13,14 Activated carbons due to porous structure exhibit very high SSA, however, due to the geometry and path constraints these pores are not fully accessible to the electrolytic ions and in most of studies 10–20% activated carbons have been utilized, those consequently constitutes low Csp (<10 μF cm−2) which is much smaller to the theoretically predicted value of specific capacitance.15 These days research efforts are centered on increasing specific capacitance of the carbon based materials by doping of hetero-atom (boron, sulphur, phosphorous, nitrogen etc.) in carbon lattice rather than enhancing it through SSA. Among the hetero-atom dopants in carbon, doping of N-doping is mostly practiced and has shown interesting properties.16,17 Doping of N-atom in carbon lattice is either carried out by chemical treatment on carbon or by pyrolysis of N containing precursors.18–24 Pyrolysis of N containing precursor offer advantages like low cost, simple preparation and on top of all the formation of homogeneously N-doped carbon. Various N-containing precursors like natural (silk,25 gelatin,26 soyabeans,27 seaweed28 etc.) and synthetic (porous melamine foam,29 metal–organic framework,30 conducting polymers like polypyrrole and polyaniline31–34) have been used.Morphologically different N-doped carbon materials like nanocages,35 spheres,36 nanotube37 etc., obtained after carbonizing polyaniline are previously reported. Among the various morphologies of N-doped carbon, nanosheets could be a potential candidate for supercapacitor electrodes because of its resemblance to graphene.38 However, synthesis of uniformly N doped carbon sheet is a challenging task.
Here in this article, to synthesize N doped carbon nanosheet soft template assisted facile route for PANI nanosheets (PNS) synthesis is utilized. These PNS were further subjected to pyrolysis at different temperatures (400–600 °C) to form nitrogen doped carbon nanosheets (NCNS). Depending upon the pyrolysis temperature these sheets were named as NCNS 400, NCNS 500, NCNS 600 respectively. The pyrolysis temperature range was very carefully chosen because at 400 °C the carbonization of a material starts and at 600 °C, the N present in a carbon lattice start disintegrating.25
As prepared NCNS powders were used to fabricate electrodes of supercapacitor cells. These cells were characterized using aqueous (1 M H2SO4) and solid electrolyte (PVA/H2SO4). Our results indicate that due to high N-atomic percentage (∼15 wt%) NCNS 400 showed superior charge storage properties due to increased pseudocapacitive contribution from N-based functionalities and high electrolyte accessibility due to the increased inter-planar distance (d-spacing).
2. Experimental section
2.1 Materials
Aniline (ANI, Merck Ltd.), ammonium persulphate (APS, Merck Ltd.), HCl (35–38%, Thomas Baker), H2SO4 (∼98%, Thomas Baker), isopropyl alcohol (IPA, SRL) and N-cetyl-N,N,N-trimethyl ammonium bromide (CTAB, Spectrochem), polyvinyl alcohol (PVA middle chain length, MERCK) used here were of AR grade.2.2 Synthesis of PNS
PANI nanosheets were synthesized through facile soft template method using CTAB as cationic surfactant. 2 mM aniline (ANI) monomer was dispersed in 20 ml of 1 M HCl containing 1.5 mM aqueous solution of CTAB. A pre-cooled solution of APS having monomer/oxidant ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was added to the above reaction mixture at once and kept under stirring for 3–4 h to ensure complete mixing. The mixture was left undisturbed overnight at 25 °C. A dark green product was formed which was collected by filtration through 0.22 μm filtration membrane and washed thoroughly with distilled water. Further, the product was washed with ethanol and dried under vacuum for 24 h. For comparison PANI is also synthesized without using CTAB surfactant in same procedure.
1 was added to the above reaction mixture at once and kept under stirring for 3–4 h to ensure complete mixing. The mixture was left undisturbed overnight at 25 °C. A dark green product was formed which was collected by filtration through 0.22 μm filtration membrane and washed thoroughly with distilled water. Further, the product was washed with ethanol and dried under vacuum for 24 h. For comparison PANI is also synthesized without using CTAB surfactant in same procedure.
2.3 Carbonization of PNS to form NCNS 400, NCNS 500 and NCNS 600
The above synthesized PNS were then carbonized in furnace at 400 °C, 500 °C and 600 °C in argon atmosphere separately. So obtained carbonized sample of PANI nanosheets were then named as NCNS 400, NCNS 500 and NCNS 600.2.4 Electrode and supercapacitor cell fabrication
10 mg powder of each sample (PNS, NCNS 400, NCNS 500 and NCNS 600) was separately subjected to ultrasonication in IPA with 10 μL of Nafion binder for 1 hour and the dispersion was deposited on pre cleaned ITO glass plates (1 cm2) followed by drying in vacuum oven at 80 °C for 24 h. The weight of the deposited film (controlled to ∼0.25 mg cm−2) was gravimetrically estimated. Solid-state supercapacitor cells were fabricated by sandwiching PVA/H2SO4 solid electrolyte membrane between two electrodes. PVA/H2SO4 gel that served as electrolyte as well as separator was synthesized by dissolving 2 g PVA powder in 20 ml solution (2 g H2SO4 + deionized water) with agitation and heating at 90 °C to obtain a clear solution. Finally, the homogeneous viscous solution (electrolyte) was poured into a Petridish and left overnight to solidify at room temperature. Aqueous supercapacitor cells were fabricated by sandwiching a Nafion membrane as separator.2.5 Characterization
Morphological features of PNS, NCNS 400, NCNS 500 and NCNS 600 were studied by high resolution transmission electron microscopy (HRTEM) using Phillips Technai T-300 and Zeiss Ultra 55 field emission scanning electron microscope (FESEM). X-ray diffraction (XRD) data of the PANIs were collected using D8 DISCOVER high resolution X-ray diffractometer and Renishaw Invia Reflex Micro-Raman spectrometer was used to record Raman spectrum. Nitrogen percentage in all four samples were estimated by using CHNSO analyser (Elementar Analysensysteme Germany, Vario Micro Cube). Electronic conductivity was measured using 6815B Keithley electrometer as described in our earlier article.25 Surface area and pore size distribution were carried out on Gemini-V, Micromeritics and Micromeritics ASAP 2020. Cyclic voltammetry (CV) and electrochemical impedance spectroscopic (EIS) investigations of the samples under discussion were carried out on electrochemical workstation CHI (604 D). Cyclic voltammetry (CV) in three electrode cell were performed using the sample film as working electrode, Ag/AgCl as reference and Pt as counter electrode. All the CVs (three cell electrode or two cell electrode) were recorded in working potential window of 0.0 V to 1.0 V. Galvanostatic charge–discharge (GCD) characteristics were recorded using PARSTAT-4000 instrument.3. Results and discussion
Scheme 1, represents the formation of various NCNS samples from PNS. Firstly, the PNS is synthesized using surfactant assisted in situ chemical polymerization. Charring of PNS samples at different temperatures in inert atmosphere results in structural changes and therefore different levels of N accommodation in host carbon lattice in achieved.25 Structural changes are expected during the carbonization process as some elements in the form of gaseous products escape from matrix and subsequently lead to restructuring in the material. When PNS undergoes heat treatment in inert atmosphere (argon) at 400 °C, exfoliation of sheets occur with slight loss of N wt%. Due to the depletion of N wt% from the material at high temperature, structural deformation takes place as depicted in Scheme 1.3.1 Structural analysis
Fig. 1(a) shows the CHN analysis to estimate N (wt%) in PNS, NCNS 400, NCNS 500 and NCNS 600. In general, N content revealed depletion from PNS to NCNS as the heating temperature increases. There drastic decrease (∼9 wt% N) from 400 °C to 600 °C, is in agreement with similar studies.25 Upon releasing such a high N (9 wt%) content, the sheet structures breaks into the fragments. UV-visible spectrum of PNS shows a peak at 270 nm belonging to the molecular conjugation (Fig. 1(b)).39 Peak at 360 nm signifies the doped emeraldine state of PANI and it attributes to the valence band electronic transition to polaron band. Slight shift in the peak position due to molecular conjugation in NCNS 400 is ascribed to loss in conjugation in PANI after the pyrolysis. Further the gradual disappearance of this peak with increase in temperature confirms loss in conjugation. Raman spectroscopic analysis (Fig. 1(c and d)) helped in identifying the structure and ordering in PNS and NCNS samples. Raman spectrum of PNS exhibited peaks at 1218, 1326, 1492 and 1572 cm−1 correspond to in-plane ring deformation, C–N+ stretching, quinoid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching and benzenoid C–C stretching correspondingly.40,41 The D phonon mode and G graphitic or E2g mode are observed at 1352 and 1573 cm−1. Peak at 1352 cm−1 arises due to the conversion of sp2 hybridized carbon into the sp3 carbon leading to the structural defect in graphitic carbon matrix whereas peak at 1573 cm−1 reveal the graphitic or ordered sp2 carbons.42 Fig. 1(d) shows the graphitic (ordered) character and the disordering in PNS and NCNS samples by means of D and G peak (ID/IG) ratio. As seen in the graph the ID/IG ratio decreases from PNS (1.0) to NCNS 400 (0.96) which signifies increase in ordering or graphitic character on heat treatment. The subsequent increase in ID/IG ratio from NCNS 500 (0.98) to NCNS 600 (1.1) indicate the increased disordering in structure due to removal of N leading to the lattice defects the carbon lattice. X-ray diffraction patterns in Fig. 1(e and f) reveal the characteristics of PANI in PNS and d-spacing of the carbonised PNS samples. The prominent peak at 2θ value of 14.5°, 19.6° and 25.5° are associated with the 001, 020 and 200 planes of emeraldine salt of PANI.43 Pyrolysed samples show characteristic peak of carbon corresponding to 002 plane at ∼21° and the d-spacing calculated from this peak are shown in Fig. 1(e). Interestingly the d-spacing shows an increase from PNS (0.33 nm) to NCNS 400 (0.45 nm) however on further increasing the temperature, a decrease in d-spacing to 0.40 nm (NCNS 500) and 0.37 nm (NCNS 600) is noticed. The d-spacing values observed through SAED (selected area electron diffraction) patterns in Fig. 2, show close proximity to the values observed in X-ray diffraction results. The minor variation could be due to the amorphous nature of the samples noticed via diffused broad bands. The first increase of d-spacing is attributed to the structural change in material from PNS to NCNS due to exfoliation. The subsequent decrease is resulted from the depletion of N from carbon lattice as it approaches to pure carbon d-spacing 0.34 nm.
N stretching and benzenoid C–C stretching correspondingly.40,41 The D phonon mode and G graphitic or E2g mode are observed at 1352 and 1573 cm−1. Peak at 1352 cm−1 arises due to the conversion of sp2 hybridized carbon into the sp3 carbon leading to the structural defect in graphitic carbon matrix whereas peak at 1573 cm−1 reveal the graphitic or ordered sp2 carbons.42 Fig. 1(d) shows the graphitic (ordered) character and the disordering in PNS and NCNS samples by means of D and G peak (ID/IG) ratio. As seen in the graph the ID/IG ratio decreases from PNS (1.0) to NCNS 400 (0.96) which signifies increase in ordering or graphitic character on heat treatment. The subsequent increase in ID/IG ratio from NCNS 500 (0.98) to NCNS 600 (1.1) indicate the increased disordering in structure due to removal of N leading to the lattice defects the carbon lattice. X-ray diffraction patterns in Fig. 1(e and f) reveal the characteristics of PANI in PNS and d-spacing of the carbonised PNS samples. The prominent peak at 2θ value of 14.5°, 19.6° and 25.5° are associated with the 001, 020 and 200 planes of emeraldine salt of PANI.43 Pyrolysed samples show characteristic peak of carbon corresponding to 002 plane at ∼21° and the d-spacing calculated from this peak are shown in Fig. 1(e). Interestingly the d-spacing shows an increase from PNS (0.33 nm) to NCNS 400 (0.45 nm) however on further increasing the temperature, a decrease in d-spacing to 0.40 nm (NCNS 500) and 0.37 nm (NCNS 600) is noticed. The d-spacing values observed through SAED (selected area electron diffraction) patterns in Fig. 2, show close proximity to the values observed in X-ray diffraction results. The minor variation could be due to the amorphous nature of the samples noticed via diffused broad bands. The first increase of d-spacing is attributed to the structural change in material from PNS to NCNS due to exfoliation. The subsequent decrease is resulted from the depletion of N from carbon lattice as it approaches to pure carbon d-spacing 0.34 nm.
 | ||
| Fig. 2 (a–d) SAED pattern of PNS, NCNS 400, NCNS 500 and NCNS 600 samples showing d-spacing and their respective planes. | ||
3.2 Microstructural analysis
 | ||
| Fig. 4 SEM micrographs of samples (a) PNS, (b) NCNS 400, (c) NCNS 500 and (d) NCNS 600 showing the significant fragmentation of the sheet morphology with increase in temperature above 400 °C. | ||
The N2 adsorption–desorption isotherms of all four samples are shown in Fig. 5(a–d). BET specific surface area shows incremental values from PNS to NCNS 600. PNS exhibits lowest specific surface area of 19.9 m2 g−1 while NCNS 400, NCNS 500 and NCNS 600 shows specific surface area of 52.18 m2 g−1, 81.02 m2 g−1 and 81.7 m2 g−1 respectively. First increase in BET specific surface area (PNS to NCNS 400) is attributed to the exfoliation of sheets as observed in TEM and SEM micrographs with increased d-spacing. Further increase in surface area corresponds to fragmentation of sheets into smaller parts. There is no significant variation observed in NCNS 500 and NCNS 600 which might be due to the decrease of d-spacing. Interestingly all the four samples show micro and mesoporous porosity. Existence of the micro and mesoporous porosity is well recognised to maximize the specific capacitance.1
3.3 Electrochemical analysis
Morphology of electrode material has profound effects on the electrochemical performance of the supercapacitor cell. In order to understand the role of surfactant CTAB studied in this research, PANI sample was synthesised without CTAB. The results presented in ESI (Fig. S1†) indicate underperformance due to the irregular morphology of PANI compared to the PNS discussed in next section. Charge storage characteristics of PNS, NCNS 400, NCNS 500 and NCNS 600 electrodes were examined by CV experiments in 3 electrode cell assembly taking 1 M H2SO4 as electrolyte (Fig. 6(a)). The specific capacitance (Csp) calculated from the voltammograms by following equation:
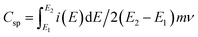 | (1) |
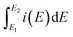 : integration of positive and negative sweep, E2 − E1: working potential window, m: mass of material on the electrode, ν: scan rate potential.
: integration of positive and negative sweep, E2 − E1: working potential window, m: mass of material on the electrode, ν: scan rate potential.
The highest Csp observed in NCNS 400 was about 564.5 F g−1 which is ∼1.5 times higher than PNS (394 F g−1). This enhancement is attributed to the increased d-spacing that helped in enhancing material utilization through electrolytic ion accessibility.44 Further the presence of nitrogen related functional groups enhanced the pseudocapacitive contribution in NCNS. Pair of redox peaks in PNS sample shows the transformation of emeraldine to pernigraniline state. While the sharp redox peak around 0.4 V to 0.6 V in NCNS 400 is assigned to the basic functionalities/reversible redox conversion of quinone to hydroquinone.45,46 The above redox peaks show a gradual decrease in current as the temperature increases from 400–600 °C. Voltammograms in Fig. 6(a) clearly signifies that the charge storage decreases with increasing temperature. This is primarily ascribed to the depleting N (wt%) that in turns lead to decreased electronic conductivity and subsequent pseudocapacitive contribution. The electrical conductivity values for all samples are as follows: 1.6 × 10−4 (PNS), 2.3 × 10−3 (NCNS 400), 2.8 × 10−5 (NCNS 500) and 4.3 × 10−6 (NCNS 600). NCNS 400 shows better conductivity due to the carbonization and better alignment of atoms. Ideally as the carbonization temperature increases conductivity of carbon increases but in case of NCNS 500 and NCNS 600 conductivity showed decrement due to depletion of nitrogen and fragmentation of sheets. The role of morphological changes in charge storage capacity, effects of agglomeration and crumbled morphology through grain boundary resistance etc. cannot be ruled out. Similar trend was observed in the solid state supercapacitor cell as the specific capacitance follow the decreasing order NCNS 400 (186 F g−1) > PNS (107.5 F g−1) > NCNS 500 (58 F g−1) > NCNS 600 (45 F g−1) calculated from the CV curves in Fig. 6(b).
In order to further investigate the charge storage characteristics in liquid and solid state supercapacitor cells, galvanostatic charge–discharge cycles at various constant current loads were recorded and the supercapacitor cell capacitance (Cm) was calculated by this formula:
The supercapacitor cell capacitance in both aqueous and solid electrolyte follows the same trend as observed in Fig. 6. It is important to state that iR-drop of a cell which corresponds to ESR (equivalent series resistance) is a crucial parameter for cell performance. In case of NCNS 400 the iR-drop is the least that demonstrates the best cell among other (Fig. 6(c and e)). The charge storage capacity of both the supercapacitor cells (liquid and solid) at different current densities are shown in Fig. 6(d and f). Decay with increasing current density is observed, however, this is less in liquid cell compared to solid state cell because of the ion diffusion. This is acceptable due to electrolyte accessibility is surface controlled process in solid state. The supercapacitor cell characteristics in both states are summarised in Table 1.
| Sample | Csp (CV)@5 mV s−1 | Cm (GCD)@0.6 A g−1 | Rs | Rct | τo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 cell (L) F g−1 | 2 cell (S) F g−1 | 2 cell (L) F g−1 | 2 cell (S) F g−1 | 2 cell (L) Ω | 2 cell (S) Ω | 2 cell (L) Ω | 2 cell (S) Ω | 2 cell (L) | 2 cell (S) | |
| a Note: L-liquid electrolyte (1 M H2SO4) and S-solid electrolyte (PVA/H2SO4). | ||||||||||
| PNS | 394 | 107.5 | 121 | 76.37 | 1.4 | 5.8 | 2.52 | 1.8 | 1.0 s | 0.05 s |
| NCNS 400 | 564.5 | 186 | 161.7 | 59.14 | 1.0 | 1.1 | 0.49 | 1.12 | 0.84 s | 0.01 s |
| NCNS 500 | 174.5 | 58 | 48.35 | 43.7 | 2.7 | 4.6 | 1.13 | 1.5 | 5.9 s | 0.84 s |
| NCNS 600 | 160 | 45 | 39.5 | 42.73 | 1.6 | 4.9 | 1.15 | 1.6 | 50 s | 55.5 s |
Frequency response characteristics of both types of supercapacitor cells are shown in Nyquist plots (Fig. 7(a–d)) and corresponding solution resistance (Rs) and charge transfer resistance (Rct) values are tabulated in the Table 1. NCNS 400 plot is nearly parallel to the imaginary axis depicting nearly ideal supercapacitor performance. Moreover, the low Rs and Rct values reflect the fast ionic and electronic charge transfer. This is expected as the high percentage of N-doping (∼15%) is known to increase the electronic conductivity of N-doped carbon sample. Additionally 2D sheet like structures with high inter planar distance offers low resistive pathway for ion conduction.25 Bode plot of the supercapacitor cells with both liquid and solid electrolyte and their corresponding relaxation time constant (τo) were shown in Fig. 7(e and f).39 Phase angle value of NCNS 400 is approaching towards the ideal electrochemical capacitor value (−90°) in both the liquid (−84.5°) and solid (−80°) electrolyte. Low phase angle of rest three samples in both electrolytes follow the same trend of decrement as shown in the capacitive value obtained from GCD and CV. Low relaxation time constant value of NCNS 400 suggest that NCNS 400 cell shows capacitive character at higher frequency. The electrolyte ions are able to penetrate deeper in NCNS 400 electrode matrix that consequently results in the fast charge and discharge of the cell.47–50
GCD cycling life tests were performed on the PNS, NCNS 400, NCNS 500 and NCNS 600 supercapacitor cells in liquid and solid electrolyte (Fig. 8(a and b)). Since conducting polymers during redox cycling show poor cyclability due to breaking of molecular chains,51 the same was reflected in PNS that showed 93.5% retention after 1000 cycles in liquid and 98.7% in solid supercapacitor cell. Improved performance in solid electrolyte signifies that electrochemical reactions are surface controlled in spite of bulk (liquid electrolyte). NCNS 400 showed consistently high performance in both the electrolytes because of the fact that it no longer contains the PANI chains and therefore no chain defects upon mass insertion and de-insertion. Relatively poor GCD cycling life in NCNS 500 and NCNS 600 is resulted from the agglomerated, crumbled, resistive morphology with high iR-drop. In order to study the power density with energy density, the Ragone plots in Fig. 8(c and d) compares the NCNS supercapacitor cells with PNS supercapacitor cell. Energy density and power density are calculated as per these equations:52
 | (2) |
 | (3) |
Low relaxation time and iR-drop strongly supports the highest energy and power delivery of NCNS 400 among all other samples. It shows high energy density of 21.6 W h kg−1 at power density of 293.91 W kg−1 in 1 M H2SO4 and 9.8 W h kg−1 at 288 W kg−1 in PVA/H2SO4 as shown in Fig. 8(c and d).
4. Conclusions
We report a simple and cost effective method to synthesize nitrogen doped carbon sheets by pyrolizing the polyaniline sheets. Our study revealed a moderate temperature (400 °C) at which polyaniline converts to carbon sheet with high (15 wt%) nitrogen doping that enhanced the electronic conductivity and the pseudocapacitance of the carbon material. Results suggest that the nitrogen doped carbon nanosheets prepared at 400 °C (NCNS 400) constitute the high performance supercapacitor cell in solid as well as electrolytes. This underperformance cycling stability of PANI nanosheets (PNS) resolved to a great extent by carbonising the PNS the nitrogen containing precursor (PNS). Although the gravimetric capacitance of PNS could be higher than the NCNS however the performance of the resulted material is improved. The study reports a facile method to obtain nitrogen doped carbon nanosheets like N doped graphenes.Acknowledgements
The authors gratefully acknowledge University of Delhi for supporting the research through R&D grant (2015-16). Financial support from DST through sponsored research (SR/S1/PC-31/2010) is gratefully acknowledged. One of the authors, V. S. especially acknowledges the SRF award (9/45(1269)/2013-EMR-I) from CSIR, INDIA.References
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- H. Jiang, L. Yang, C. Li, C. Yan, P. S. Lee and J. Ma, Energy Environ. Sci., 2011, 4, 1813–1819 CAS.
- Q. Qu, S. Yang and X. Feng, Adv. Mater., 2011, 23, 5574–5580 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294–1301 CAS.
- J. Bae, M. K. Song, Y. J. Park, J. M. Kim, M. Liu and Z. L. Wang, Angew. Chem., Int. Ed., 2011, 50, 1683–1687 CrossRef CAS PubMed.
- J. R. Miller, R. A. Outlaw and B. C. Holloway, Science, 2010, 329, 1637–1639 CrossRef CAS PubMed.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- V. Sahu, S. Shekhar, P. Ahuja, G. Gupta, S. K. Singh, R. K. Sharma and G. Singh, RSC Adv., 2013, 3, 3917–3924 RSC.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- B. Xu, F. Wu, R. Chen, G. Cao, S. Chen, Z. Zhou and Y. Yang, Electrochem. Commun., 2008, 10, 795–797 CrossRef CAS.
- G. Gryglewicz, J. Machnikowski, E. Lorenc-Grabowska, G. Lotab and E. Frackowiak, Electrochim. Acta, 2005, 50, 1197–1206 CrossRef CAS.
- J. P. Paraknowitsch and A. Thomas, Energy Environ. Sci., 2013, 6, 2839–2855 CAS.
- S. Yang, L. Zhi, K. Tang, X. Feng, J. Maier and K. Mullen, Adv. Funct. Mater., 2012, 22, 3634–3640 CrossRef CAS.
- D. Hulicova-Jurcakova, M. Kodama, S. Shiraishi, H. Hatori, Z. H. Zhu and G. Q. Lu, Adv. Funct. Mater., 2009, 19, 1800–1809 CrossRef CAS.
- K. Jurewicza, R. Pietrzakb, P. Nowickib and H. Wachowska, Electrochim. Acta, 2008, 53, 5469–5475 CrossRef.
- L. Wang, Z. Gao, J. Chang, X. Liu, D. Wu, F. Xu, Y. Guo and K. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 20234–20244 CAS.
- E. J. Ra, E. Raymundo-Pinero, Y. H. Lee and F. Beguin, Carbon, 2009, 47, 2984–2992 CrossRef CAS.
- S. Maldonado, S. Morin and K. J. Stevenson, Carbon, 2006, 44, 1429–1437 CrossRef CAS.
- D. Hulicova, J. Yamashita, Y. Soneda, H. Hatori and M. Kodama, Chem. Mater., 2005, 17, 1241–1247 CrossRef CAS.
- G. Lota, K. Lota and E. Frackowiak, Electrochem. Commun., 2007, 9, 1828–1832 CrossRef CAS.
- V. Sahu, S. Grover, B. Tulachan, M. Sharma, G. Srivastava, M. Roy, M. Saxena, N. Sethy, K. Bhargava, D. Philip, H. Kim, G. Singh, S. K. Singh, M. Das and R. K. Sharma, Electrochim. Acta, 2015, 160, 244–253 CrossRef CAS.
- B. Xu, S. Hou, G. Cao, F. Wu and Y. Yang, J. Mater. Chem., 2012, 22, 19088–19093 RSC.
- C. Long, L. Jiang, X. Wu, Y. Jiang, D. Yang, C. Wang, T. Wei and Z. Fan, Carbon, 2015, 93, 412–420 CrossRef CAS.
- E. Raymundo-Pinero, M. Cadek and F. Beguin, Adv. Funct. Mater., 2009, 19, 1032–1039 CrossRef CAS.
- L. Shen, J. Wang, G. Xu, H. Li, H. Dou and X. Zhang, Adv. Energy Mater., 2014, 5, 1400977 Search PubMed.
- J.-W. Jeon, R. Sharma, P. Meduri, B. W. Arey, H. T. Schaef, J. L. Lutkenhaus, J. P. Lemmon, P. K. Thallapally, M. I. Nandasiri, B. P. McGrail and S. K. Nune, ACS Appl. Mater. Interfaces, 2014, 6, 7214–7222 CAS.
- Z. Rozlivkova, M. Trchová, M. Exnerova and J. Stejskal, Synth. Met., 2011, 161, 1122–1129 CrossRef CAS.
- C. Wu, X. Wang, B. Ju, L. Jiang, H. Wu, Q. Zhao and L. Yi, J. Power Sources, 2013, 227, 1–7 CrossRef CAS.
- S. Mentus, G. Ciric-Marjanovic, M. Trchova and J. Stejskal, Nanotechnology, 2009, 20, 245601 CrossRef PubMed.
- G. Ciric-Marjanovic, I. Pasti, N. Gavrilov, A. Janosevic and S. Mentus, Chem. Pap., 2013, 67, 781–813 CAS.
- Y. Tan, C. Xu, G. Chen, Z. Liu, M. Ma, Q. Xie, N. Zheng and S. Yao, ACS Appl. Mater. Interfaces, 2013, 5, 2241–2248 CAS.
- J. Han, G. Xu, B. Ding, J. Pan, H. Dou and D. R. MacFarlane, J. Mater. Chem. A, 2014, 2, 5352–5357 CAS.
- M. Yang, B. Cheng, H. Song and X. Chen, Electrochim. Acta, 2010, 55, 7021–7027 CrossRef CAS.
- A. Ambrosi, C. K. Chua, A. Bonanni and M. Pumera, Chem. Rev., 2014, 114, 7150–7188 CrossRef CAS PubMed.
- S. Grover, S. Goel, V. Sahu, G. Singh and R. K. Sharma, ACS Sustainable Chem. Eng., 2015, 3, 1460–1489 CrossRef CAS.
- M. Ginic-Markovic, J. G. Matisons, R. Cervini, G. P. Simon and P. M. Fredericks, Chem. Mater., 2006, 18, 6258–6265 CrossRef CAS.
- Z. Wei, M. Wan, T. Lin and L. Dai, Adv. Mater., 2003, 15, 136–139 CrossRef CAS.
- V. Sahu, S. Shekhar, R. K. Sharma and G. Singh, ACS Appl. Mater. Interfaces, 2015, 7, 3110–3116 CAS.
- Y. Zhao, G.-S. Tang, Z.-Z. Yu and J.-S. Qi, Carbon, 2012, 50, 3064–3073 CrossRef CAS.
- M. M. Hantel, T. Kaspar, R. Nesper, A. Wokaun and R. Kotz, Chem.–Eur. J., 2012, 18, 9125–9136 CrossRef CAS PubMed.
- D. Hulicova-Jurcakova, M. Seredych, G. Q. Lu and T. J. Bandosz, Adv. Funct. Mater., 2009, 19, 438–447 CrossRef CAS.
- G. Lota, B. Grzyb, H. Machnikowska, J. Machnikowski and E. Frackowiak, Chem. Phys. Lett., 2005, 404, 53–58 CrossRef CAS.
- R. Farmal, M. Deraman, Awitdrus, I. A. Talib, R. Omar, J. G. Manjunatha, M. M. Ishak, N. H. Basri and B. N. M. Dolah, Int. J. Electrochem. Sci., 2013, 8, 257–273 Search PubMed.
- C. Portet, P. L. Taberna, P. Simon, E. Flahaut and C. Laberty-Robert, Electrochim. Acta, 2005, 50, 4174–4181 CrossRef CAS.
- L. Zhang, F. Zhang, X. Yang, G. Long, Y. Wu, T. Zhang, K. Leng, Y. Huang, Y. Ma, A. Yu and Y. Chen, Sci. Rep., 2013, 3, 1408, DOI:10.1038/srep01408.
- A. Bello, F. Barzegar, D. Momodu, J. Dangbegnon, F. Taghizadeh, M. Fabiane and N. Manyala, J. Power Sources, 2015, 273, 305–311 CrossRef CAS.
- R. K. Sharma, A. C. Rastogi and S. B. Desu, Electrochem. Commun., 2008, 10, 268–272 CrossRef CAS.
- V. Sahu, S. Goel, R. K. Sharma and G. Singh, Nanoscale, 2015, 7, 20642–20651 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00068a |
| This journal is © The Royal Society of Chemistry 2016 |