Preparation of binder-free CuO/Cu2O/Cu composites: a novel electrode material for supercapacitor applications
Panpan Xua,
Jijun Liua,
Tong Liua,
Ke Yea,
Kui Chenga,
Jinling Yina,
Dianxue Caoa,
Guiling Wang*a and
Qiang Li*b
aKey Laboratory of Superlight Materials and Surface Technology of Ministry of Education, College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin, 150001, P. R. China. E-mail: wangguiling@hrbeu.edu.cn; Fax: +86-451-82589036; Tel: +86-451-82589036
bKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Materials Science, Heilongjiang University, Harbin, 150080, P. R. China. E-mail: liqiang@hlju.edu.cn
First published on 4th March 2016
Abstract
Cuprous(I) oxide (Cu2O) carries high theoretical specific capacitance (2247.6 F g−1), however, the amount of research about the supercapacitive performance of Cu2O is relatively small compared with other transition metal oxides. A composite of metal and metal oxide could improve the electrochemical performance efficiently. In this work, the results of XRD and XPS demonstrate that CuO/Cu2O/Cu is prepared successfully via a facile, eco-friendly, one-step template-free growth process. SEM figures show that cubic CuO/Cu2O/Cu uniformly and densely covers a skeleton of nickel foam. The binder-free CuO/Cu2O/Cu electrode exhibits excellent supercapacitive performance with a high specific capacitance of 878 F g−1 at a current density of 5 mA cm−2 (1.67 A g−1), when the current density is enlarged ten times (50 mA cm−2 (16.7 A g−1)), the specific capacitance still remains at 545 F g−1. Furthermore, we have first successfully constructed a CuO/Cu2O/Cu//AC asymmetric supercapacitor, which can achieve an energy density of 42 W h kg−1 at a power density of 0.44 kW kg−1. The good electrochemical performance and simple accessibility prove that the as-prepared CuO/Cu2O/Cu/NF electrode has a potential application in electrochemical capacitors.
1. Introduction
Supercapacitors have attracted great attention1,2 due to the merits of longer cycle life, and higher power density compared with other energy storage devices,3–5 such as fuel cells, batteries, and conventional capacitors.According to the mechanism of electric storage, there are two kinds of supercapacitors: (i) electrochemical double layer capacitors (EDLCs) based on carbon materials (activated carbon, graphite, nanotubes (CNTs), etc.) as the electrode, storing energy by adsorption of charges at the surface between the electrode and electrolyte, which usually show lower capacitance and (ii) faradic capacitors based on transition metal oxide or conducting polymer materials as the electrode, storing energy by redox reactions, which always display considerably higher specific capacitance.6 Because of the features of lower cost, larger abundance, and lower toxicity, many transition metal oxides, such as MnOx,7,8 Ni(OH)2,9,10 Co(OH)2,11,12 CuO,13–15 Fe2O3,16–18 V2O5,19,20 and SnO2,21,22 have been widely investigated as electrode materials for supercapacitors. However, there is another neglected metal oxide in this field, Cu2O, which is extensively attractive due to its promising applications in carbon monoxide (CO) oxidation,23 photocatalysts,24 solar-driven water splitting,25 solar energy conversion,26 negative electrode materials for lithium-ion batteries27 and antibacterial activity.28 To the best of our knowledge, research about the systematic investigation of the supercapacitor performance of Cu2O is rare and there are not many papers on fabricating an asymmetric supercapacitor with Cu2O as the positive electrode (as summarized in Table 1). Most importantly, the theoretical capacitance of Cu2O that can be achieved is as high as 2247.6 F g−1, calculated from the equation: n × F/m × V,29 where n is the number of electrons transferred in the redox reaction, F is the Faraday constant, m is the molecular weight of the metal oxide and V is the charging voltage range, which predicts the potential application of Cu2O in a supercapacitor. Therefore, further investigations, such as into easier preparation methods, nanoscale morphology, and better supercapacitor performance, are needed for a sensible assessment of its actual potential.
| Materials | Structure | Current density | Cs (F g−1) | Ref. |
|---|---|---|---|---|
| Cu2O | Powder | 0.1 A g−1 | 144 | 30 |
| Cu2O@Cu | Nanoneedle array | 2.9 A g−1 | 510.2 | 41 |
| Cu2O/Ni | — | 500 mV s−1 | 427 | 46 |
| Cu2O/RGO | Cubic particles | 1 A g−1 | 98.5 | 34 |
| Cu2O/RGO | Powder | 100 mA g−1 | 31 | 31 |
| Cu2O/CuO/RGO | Powder | 1 A g−1 | 173.4 | 32 |
| Cu/Cu2O/graphene | Powder | 0.1 A g−1 | 257 | 33 |
| Cu2O/Cu | Cube on nickel foam | 5 mA cm−2 (1.67 A g−1) | 878 | This work |
As seen in Table 1, the common preparation technique for Cu2O electrodes is to firstly synthesise pure Cu2O30 or Cu2O and carbonous material composites31–34 and then smear the electrochemically active materials with a conductive agent and binder onto the current collector. Compared to the above synthesis method, active materials directly grown on conductive substrates are more beneficial to improving supercapacitor performance, because the design of active materials directly grown on a three-dimensional current collector could efficiently facilitate the transport of electrons and ions, improving the utilization of active materials and substantially enhancing the energy density and power density. The most commonly used substrate in supercapacitors and batteries is nickel foam due to its porous architecture, large surface area, good electric conductivity and excellent stability in various kinds of liquid electrolytes.
Herein, we report a successful preparation of self-supported cubic cuprous oxide and copper composites on nickel foam (CuO/Cu2O/Cu/NF) via a simple template-free hydrothermal method. We characterized the microstructure of the electrode and investigated systematically the electrochemical performance. The CuO/Cu2O/Cu/NF electrode displays excellent supercapacitor properties. Besides, we firstly fabricate the asymmetric supercapacitor using the CuO/Cu2O/Cu/NF as the positive electrode and activated carbon (AC) as the negative electrode, which achieves a high energy density of 42 W h kg−1. This indicates that the future of CuO/Cu2O/Cu/NF in practical applications for energy storage is promising.
2. Experimental
2.1. Preparation and characterization of the positive electrode
All of the chemicals were of analytical grade and were used without further purification. 4.832 g of Cu(NO3)2·3H2O was first dissolved in 60 mL of deionized water under continuous magnetic stirring to form a homogeneous solution. Then, 1.2 g of CO(NH2)2 (urea) was added into the above solution and the mixture was stirred for another 15 min to obtain a homogeneous solution, which was then transferred into a Teflon-lined autoclave with a volume of 80 mL. A piece of nickel foam (30 mm × 50 mm × 1.5 mm, 110 PPI, 320 g m−2, Changsha Lyrun Material Co., Ltd. China) was degreased with acetone, etched with 6.0 mol dm−3 HCl for 5 min, rinsed with deionized water and then immersed into the above autoclave. The autoclave was first purged with N2 to remove oxygen and was heated at 140 °C for 14 h to induce the direct growth of CuO/Cu2O/Cu composites on the nickel foam. After cooling down naturally to room temperature, the electrode material was picked out, washed with deionized water and dried in a vacuum oven at 60 °C. The scheme is briefly shown in Scheme 1.The morphology was examined using a scanning electron microscope (SEM, JEOL JSM-6480) and transmission electron microscope (TEM, FEI Teccai G2 S-Twin, Philips). The crystallographic phases of all of the samples were investigated using an X-ray diffractometer (XRD, Rigaku TTR III) with Kα radiation (λ = 0.1506 nm) at a scan rate of 10° min−1 with a step width of 0.02°.
2.2. Electrochemical measurements
Electrochemical tests were carried out in a conventional three-electrode electrochemical cell using a computerized potentiostat (VMP3/Z Bio-Logic) controlled by the EC-lab software. The as-prepared electrode (1 × 1 cm2 nominal planar area) acted as the working electrode, platinum foil (1 × 2 cm2) served as the counter electrode, and a saturated calomel electrode (SCE) was used as the reference electrode. The asymmetric supercapacitor, using the CuO/Cu2O/Cu/NF nanosheet as the positive and AC as the negative electrode, was tested in a two-electrode electrochemical cell. The cycle life tests were conducted on a Land battery program-control test system. All of the electrochemical measurements were performed in 6.0 mol dm−3 KOH electrolyte. The solutions were made with analytical grade chemical reagents and Milli-Q water (18 MΩ cm, Millipore). EIS measurements were performed by applying an alternating voltage with a 5 mV amplitude in a frequency range from 0.01 Hz to 100 kHz at the open circuit potential.3. Results and discussion
3.1. Characterization of electrodes
The crystal structure of the electrode was firstly studied via XRD analysis. As can be seen in Fig. 1a, the peaks located at 43.5, 50.4 and 74.2 can be ascribed to the (111), (200) and (220) planes of metallic Cu (JCPDS file no. 03-1015), respectively. The diffraction peaks at 29.5, 36.4, 42.3, 61.3, 73.5 and 77.3 can be attributed to the (110), (111), (200), (220), (311) and (222) crystal planes of cubic Cu2O (JCPDS file no. 78-2076), respectively. The position of the strongest peak corresponds to the (111) crystal plane of Cu2O, indicating the [111] preferential orientation.35–37 Fig. 1b shows the structure of the (111) surface of Cu2O, the ordered pore framework between Cu and O supplies electrolyte accessible channels, ensuring more effective reduction oxidation reactions to supply capacitance, resulting in considerably increased specific capacitance and a decrease in concentration polarization at a high current, leading to improved power density.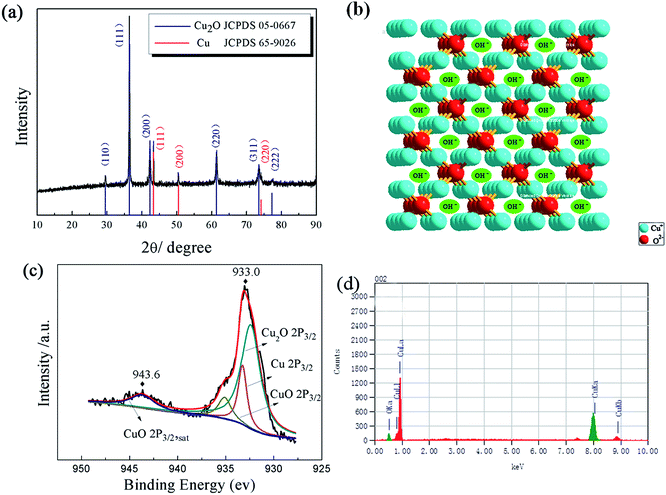 | ||
| Fig. 1 (a) XRD patterns of the CuO/Cu2O/Cu/NF electrode, (b) structure of the Cu2O (111) crystal plane, (c) XPS spectrum of Cu 2p, and (d) EDS images of the CuO/Cu2O/Cu/NF electrode. | ||
XPS was performed in the Cu 2p region to further identify the valence state of the Cu in the composite and the spectra were fitted in Fig. 1c. The main peak at the binding energy of 933.0 eV and the satellite peak at 943.6 eV were used for decomposition of the Cu peak. The broad Cu 2p3/2 peak at 933.0 eV can be divided into three peaks (932.46, 933.24 and 935.1 eV), corresponding to Cu2O, Cu and CuO,38,39 respectively. The reason for the existence of CuO may be due to the quite small fraction of superficial oxidation during sample drying, because no corresponding peaks for CuO are observed in the XRD pattern.39,40 It is acceptable that the result from the XPS is in accordance with the XRD, further indicating that the composite of Cu2O and Cu has been prepared successfully.
In addition, the EDS (Fig. 1d) reveals that the molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O is 69.5
O is 69.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.5 with a chemical formula of Cu2.28O, demonstrating that the primary phase of the CuO/Cu2O/Cu composites is Cu2O, which matches well with the results of the XRD and XPS. It should be pointed out that metallic Cu, as a perfect electric conductor, favors the transportation of electrons, improving the utilization of active materials, achieving substantially high specific capacitance.
30.5 with a chemical formula of Cu2.28O, demonstrating that the primary phase of the CuO/Cu2O/Cu composites is Cu2O, which matches well with the results of the XRD and XPS. It should be pointed out that metallic Cu, as a perfect electric conductor, favors the transportation of electrons, improving the utilization of active materials, achieving substantially high specific capacitance.
Fig. 2a is a SEM image of the nickel foam substrate, showing a three dimensional network structure supplying a large surface area for supporting active materials. Fig. 2b is a SEM image at low magnification, showing that CuO/Cu2O/Cu forms uniform cubes and densely covers the skeleton surface of the nickel foam. At high magnification (Fig. 2c), the CuO/Cu2O/Cu cubes cross over each other forming a coarse surface, which could increase the contact area of the electrode and electrolyte, improving the specific capacitance. Fig. 2d shows a TEM image, in which we can clearly see that the edge length of the cube is about 1.75 μm. In the magnified figures, the lattice spacings measured are about 0.208 nm and 0.246 nm, which fit close to the (111) crystal plane of Cu and (111) crystal plane of Cu2O (Fig. 2e), respectively. Definitely, the direct contact of the CuO/Cu2O/Cu composite to the current collector (nickel foam) avoids the conductive additive agent and polymer binder, which facilitate the transportation of electrons and ions, making the most use of the active materials, guaranteeing effective and rapid oxidation and reduction reactions to provide capacitance.
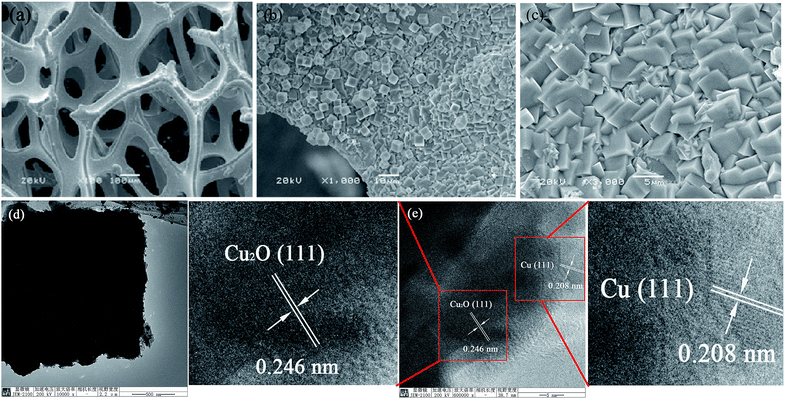 | ||
| Fig. 2 SEM images of (a) nickel foam, and (b) and (c) the CuO/Cu2O/Cu/NF electrode. (d) TEM image of Cu2O/Cu, and (e) HRTEM of CuO/Cu2O/Cu. | ||
3.2. Electrochemical properties of the electrodes
The CuO/Cu2O/Cu/NF as a positive electrode was investigated first via three-electrode measurements in 6 M KOH.The CV curves of the CuO/Cu2O/Cu/NF electrode at different scan rates are shown in Fig. 3a. An obvious pair of redox peaks are clearly seen, indicating that the faradic reactions of Cu2O when the electrode is charged and discharged is reversible and continuous. According to literature reporting on the meaning of redox peaks of copper oxide or copper hydroxide in KOH electrolytes,29,32,41–44 the charge storage mechanism of the CuO/Cu2O/Cu/NF electrode in the potential range of 0–0.5 V is suggested to be as follows:
| ½Cu2O + OH− ⇌ CuO + ½H2O + e− | (1) |
 | ||
| Fig. 3 (a) CV curves of the CuO/Cu2O/Cu/NF electrode at different scan rates and (b) peak current as a function of square root of scan rate. | ||
As the scan rate is increased, the redox peaks shift to high and low potentials, respectively, implying the quasi-reversible feature of the redox reactions. In general, the peak current ratio ipa/ipc in the CV curves is a useful judgement of reversibility. This ratio would be unity under ideal conditions. For our studied systems, the value is 1.07, approximately unity, at a scan rate of 5 mV s−1, demonstrating an excellent reversibility of electrochemical processes occurring in Cu2O, as given by eqn (1).
The peak currents of ipa (anodic peak current) and ipc (cathodic peak) against ν1/2 are shown in Fig. 3b. It is widely known that we can deduce the electrochemical reaction mechanism from the following relationship: i = aνb (b = 1.0 indicates a solid-phase diffusion controlled process; b = 0.5 indicates a surface confined charge transfer process). As can be seen, the two better linear relationships between scan rate and peak current, of which the regression equations are ipa (mA) = 30.36ν1/2 (mV s−1) − 19.41 (R2 = 0.999) and ipc (mA) = −24.83ν1/2 (mV s−1) + 9.19 (R2 = 0.999), indicate that the redox reactions of the CuO/Cu2O/Cu/NF electrode are diffusion controlled processes. The remaining double-layer capacitance and background current lead to the non-zero intercepts on the fitting line.45
Additionally, Fig. 4a shows GCD curves at current densities from 5 mA cm−2 to 50 mA cm−2. According to the CV curves (Fig. 3a), to avoid the oxygen evolution destroying the electrode structure in the process of charging, we chose 0–0.4 V as the GCD potential range. Obviously, the charge time is equal to the corresponding discharge time for all of the curves, indicating that the electricity charged can be released completely, which demonstrates the perfect reversibility of the CuO/Cu2O/Cu/NF electrode. The area specific capacitance (Cs (F cm−2)) and mass specific capacitance (Cm (F g−1)) are calculated based on the following equations:
 | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31). In addition, a high specific capacitance of 545 F g−1 at the not too high current density of 50 mA cm−2 (16.7 A g−1) is scarce for transition metal oxide materials, such as MnO2 (381 F g−1 at 10 A g−1),47 Co(OH)2 (385 F g−1 at 16 A g−1),48 and NiO (368 F g−1 at 20 A g−1).49 The superior capacitance can be rationalized as follows. First, the 3D continuous skeleton of nickel foam supplies a large surface area for supporting more active material to produce capacitance and the 3D porous structure provides efficient electrolyte diffusion channels for oxidation and reduction reactions. Second, the cubic CuO/Cu2O/Cu directly grown on nickel foam serving as an integrated electrode without a conductive agent and binder could form an expedited transportation path for electronics, reducing electrochemical polarization. Third, the interlaced cubic CuO/Cu2O/Cu expands the contact area of the electrolyte and active materials, improving the utilization of active materials. Furthermore, the existence of metallic Cu could definitely associate the transportation of electrons in the composite. Therefore, considering the easy one-step template-free hydrothermal synthesis method and the perfect electrochemical performance, our work is meaningful to the research into Cu2O as a supercapacitor electrode.
31). In addition, a high specific capacitance of 545 F g−1 at the not too high current density of 50 mA cm−2 (16.7 A g−1) is scarce for transition metal oxide materials, such as MnO2 (381 F g−1 at 10 A g−1),47 Co(OH)2 (385 F g−1 at 16 A g−1),48 and NiO (368 F g−1 at 20 A g−1).49 The superior capacitance can be rationalized as follows. First, the 3D continuous skeleton of nickel foam supplies a large surface area for supporting more active material to produce capacitance and the 3D porous structure provides efficient electrolyte diffusion channels for oxidation and reduction reactions. Second, the cubic CuO/Cu2O/Cu directly grown on nickel foam serving as an integrated electrode without a conductive agent and binder could form an expedited transportation path for electronics, reducing electrochemical polarization. Third, the interlaced cubic CuO/Cu2O/Cu expands the contact area of the electrolyte and active materials, improving the utilization of active materials. Furthermore, the existence of metallic Cu could definitely associate the transportation of electrons in the composite. Therefore, considering the easy one-step template-free hydrothermal synthesis method and the perfect electrochemical performance, our work is meaningful to the research into Cu2O as a supercapacitor electrode.
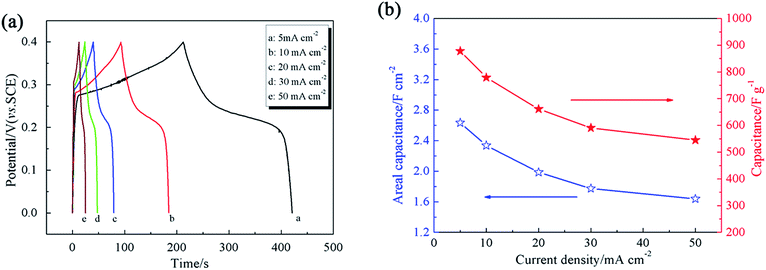 | ||
| Fig. 4 (a) GCD curves of the CuO/Cu2O/Cu/NF electrode at different current densities and (b) variation of specific capacitance as a function of current density. | ||
Fig. 5a presents the cycling stability of the CuO/Cu2O/Cu/NF electrode which was performed at a current density of 10 mA cm−2. The coulombic efficiency could be calculated via the equation as follows:
 | (4) |
The representative CV curves for the electrode are shown in Fig. 5b. The area enclosed by the CV curve after the 8000th cycle is much smaller than that for the first cycle of the electrode, but is overlapping with that of the 1000th, which is in accordance with the result of Fig. 5a.
EIS is tested to evaluate the reaction kinetics. The Nyquist plots of the fresh CuO/Cu2O/Cu/NF electrode and the electrode after 8000 charge/discharge cycles are shown in Fig. 6 and were measured at open circuit potential. Both plots show two distinct sections: in the high frequency region, there is a semicircle, of which the diameter represents the charge transfer resistance Rct (Ω) and the calculated Rct of the CuO/Cu2O/Cu/NF electrode before and after cycling is 0.05 Ω cm−2 and 0.15 Ω cm−2, respectively; and a straight line with a slope around 75°, exhibiting the features of the supercapacitor. The above results indicate that the CuO/Cu2O/Cu/NF electrode is a suitable supercapacitor electrode material.
3.3. Electrochemical properties of the asymmetric supercapacitor
We first fabricated the asymmetric supercapacitor by utilizing CuO/Cu2O/Cu/NF and AC as positive and negative electrodes, respectively. The electrochemical performance of the asymmetric supercapacitor CuO/Cu2O/Cu/NF//AC was investigated via two-electrode measurements in 6 M KOH.The CV curves at various scan rates of the asymmetric supercapacitor are shown in Fig. 6a. Because of the mutual effect of the positive and negative electrodes, the operation potential window could achieve as high as 1.6 V. Unlike the CV feature of the CuO/Cu2O/Cu/NF electrode, owing to the influence of the electrochemical double layer of the negative electrode on the whole device, the device exhibits a quasi-rectangular CV shape. With the increasing scan rate, the shape of the CV curves remain unaltered, which demonstrates the high rate capability of the device. The constant current charge–discharge curves are shown in Fig. 6b. Similarly, the DCD curves display a triangle-like shape, rather than an obvious plateau, which is well consistent with the CV curves. In addition, the good symmetry of all of the charge and discharge curves indicates the excellent electrochemical reversibility of the device.
The specific capacitance (Cm (F g−1)), specific energy (Em (W h kg−1)), and specific power (Pm (kW kg−1)) were calculated according to the following equations:
 | (5) |
 | (6) |
 | (7) |
| Current density (mA cm−2) | Discharging time (s) | Specific capacitance (F g−1) | Specific energy (W h kg−1) | Specific power (kW kg−1) |
|---|---|---|---|---|
| 5 | 340 | 117 | 42 | 0.44 |
| 10 | 150 | 104 | 37 | 0.89 |
| 20 | 62 | 86 | 30 | 1.78 |
| 30 | 33 | 69 | 25 | 2.67 |
| 50 | 14 | 50 | 18 | 4.4 |
The cycling-life test was performed for the CuO/Cu2O/Cu/NF//AC asymmetric supercapacitor via constant current charge–discharge at 20 mA cm−2 (Fig. 6d). As expected, the specific capacitance kept a similar trend compared with that of the single CuO/Cu2O/Cu/NF electrode, decreasing first and remaining stable later after 5000 repeated charge–discharge cycles. The final achieved specific capacitance was 77% of the initial value and the coulombic efficiency remained at about 100%. These results indicate that CuO/Cu2O/Cu/NF is promising for electrochemical applications as an efficient electrode material.
4. Conclusions
In conclusion, we successfully prepared cubic cuprous oxide and copper composites (CuO/Cu2O/Cu) free-standing on nickel foam via a simple hydrothermal method. The CuO/Cu2O/Cu/NF electrode in 6 mol dm−3 KOH electrolyte displays a superior electrochemical performance with a specific capacitance of 2.635 F cm−2 (878 F g−1) at a current density of 5 mA cm−2 (1.67 A g−1). Furthermore, the fabricated asymmetric supercapacitor can achieve a wide potential window of 0–1.6 V with a high energy density of 42 kW h kg−1 at a power density of 0.44 kW kg−1 and still remains at 18 kW h kg−1 at a high power density of 4.4 kW kg−1. These encouraging studies demonstrate the practical value of the low cost CuO/Cu2O/Cu material in supercapacitors.Acknowledgements
We gratefully acknowledge the financial support of this research from the National Natural Science Foundation of China (51572052, 21403044 and 51472077), the Heilongjiang Postdoctoral Fund (LBH-Z14054), and the Fundamental Research Funds for the Central Universities (HEUCF20151004), Program for New Century Excellent Talents in University (NCET-13-0779).Notes and references
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238–1251 CAS.
- X. Zhao, B. M. Sanchez, P. J. Dobson and P. S. Grant, Nanoscale, 2011, 3, 839–855 RSC.
- X. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- H. Wang and H. Dai, Chem. Soc. Rev., 2013, 42, 3088–3113 RSC.
- T. Xue, C.-L. Xu, D.-D. Zhao, X.-H. Li and H.-L. Li, J. Power Sources, 2007, 164, 953–958 CrossRef CAS.
- G. Zhu, H. Li, L. Deng and Z.-H. Liu, Mater. Lett., 2010, 64, 1763–1765 CrossRef CAS.
- O. Ghodbane, F. Ataherian, N.-L. Wu and F. Favier, J. Power Sources, 2012, 206, 454–462 CrossRef CAS.
- D.-D. Zhao, S.-J. Bao, W.-J. Zhou and H.-L. Li, Electrochem. Commun., 2007, 9, 869–874 CrossRef CAS.
- H. Jiang, T. Zhao, C. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818–3823 RSC.
- L. Cao, F. Xu, Y. Y. Liang and H. L. Li, Adv. Mater., 2004, 16, 1853–1857 CrossRef CAS.
- V. Gupta, T. Kusahara, H. Toyama, S. Gupta and N. Miura, Electrochem. Commun., 2007, 9, 2315–2319 CrossRef CAS.
- G. Wang, J. Huang, S. Chen, Y. Gao and D. Cao, J. Power Sources, 2011, 196, 5756–5760 CrossRef CAS.
- Y.-K. Hsu, Y.-C. Chen and Y.-G. Lin, J. Electroanal. Chem., 2012, 673, 43–47 CrossRef CAS.
- J. Huang, H. Wu, D. Cao and G. Wang, Electrochim. Acta, 2012, 75, 208–212 CrossRef CAS.
- P. M. Kulal, D. P. Dubal, C. D. Lokhande and V. J. Fulari, J. Alloys Compd., 2011, 509, 2567–2571 CrossRef CAS.
- X. Xia, Q. Hao, W. Lei, W. Wang, D. Sun and X. Wang, J. Mater. Chem., 2012, 22, 16844–16850 RSC.
- M.-S. Wu, R.-H. Lee, J.-J. Jow, W.-D. Yang, C.-Y. Hsieh and B.-J. Weng, Electrochem. Solid-State Lett., 2009, 12, A1–A4 CrossRef CAS.
- A. Ghosh, E. J. Ra, M. Jin, H.-K. Jeong, T. H. Kim, C. Biswas and Y. H. Lee, Adv. Funct. Mater., 2011, 21, 2541–2547 CrossRef CAS.
- G. Wee, H. Z. Soh, Y. L. Cheah, S. G. Mhaisalkar and M. Srinivasan, J. Mater. Chem., 2010, 20, 6720–6725 RSC.
- R. K. Selvan, I. Perelshtein, N. Perkas and A. Gedanken, J. Phys. Chem. C, 2008, 112, 1825–1830 CAS.
- W. Chen, R. B. Rakhi, L. Hu, X. Xie, Y. Cui and H. N. Alshareef, Nano Lett., 2011, 11, 5165–5172 CrossRef CAS PubMed.
- Q. Hua, T. Cao, H. Bao, Z. Jiang and W. Huang, ChemSusChem, 2013, 6, 1966–1972 CrossRef CAS PubMed.
- D. Barreca, G. Carraro, V. Gombac, A. Gasparotto, C. Maccato, P. Fornasiero and E. Tondello, Adv. Funct. Mater., 2011, 21, 2611–2623 CrossRef CAS.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10, 456–461 CrossRef CAS PubMed.
- R. N. Briskman, Sol. Energy Mater. Sol. Cells, 1992, 27, 361–368 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- H. Pang, F. Gao and Q. Lu, Chem. Commun., 2009, 1076–1078, 10.1039/b816670f.
- B. Vidhyadharan, I. I. Misnon, R. A. Aziz, K. P. Padmasree, M. M. Yusoff and R. Jose, J. Mater. Chem. A, 2014, 2, 6578–6588 CAS.
- L. Chen, Y. Zhang, P. Zhu, F. Zhou, W. Zeng, D. D. Lu, R. Sun and C. Wong, Sci. Rep., 2015, 5, 9672 CrossRef PubMed.
- B. Li, H. Cao, G. Yin, Y. Lu and J. Yin, J. Mater. Chem., 2011, 21, 10645–10648 RSC.
- K. Wang, X. Dong, C. Zhao, X. Qian and Y. Xu, Electrochim. Acta, 2015, 152, 433–442 CrossRef CAS.
- Y. Li, Q. Wang, P. Liu, X. Yang, G. Du and Y. Liu, Ceram. Int., 2015, 41, 4248–4253 CrossRef CAS.
- X. Dong, K. Wang, C. Zhao, X. Qian, S. Chen, Z. Li, H. Liu and S. Dou, J. Alloys Compd., 2014, 586, 745–753 CrossRef CAS.
- L. Kim, J. Kim, D. Jung, C.-Y. Park, C.-W. Yang and Y. Roh, Thin Solid Films, 2000, 360, 154–158 CrossRef CAS.
- W. Gong, J.-F. Li, X. Chu, Z. Gui and L. Li, Acta Mater., 2004, 52, 2787–2793 CrossRef CAS.
- J. Q. Hu, Y. Bando, J. H. Zhan, Y. B. Li and T. Sekiguchi, Appl. Phys. Lett., 2003, 83, 4414–4416 CrossRef CAS.
- S. Shi, D. Gao, Q. Xu, Z. Yang and D. Xue, CrystEngComm, 2015, 17, 2118–2122 RSC.
- B. Zhou, Z. Liu, H. Wang, Y. Yang and W. Su, Catal. Lett., 2009, 132, 75–80 CrossRef CAS.
- J. J. Teo, Y. Chang and H. C. Zeng, Langmuir, 2006, 22, 7369–7377 CrossRef CAS PubMed.
- C. Dong, Y. Wang, J. Xu, G. Cheng, W. Yang, T. Kou, Z. Zhang and Y. Ding, J. Mater. Chem. A, 2014, 2, 18229–18235 CAS.
- P. Xu, K. Ye, M. Du, J. Liu, K. Cheng, J. Yin, G. Wang and D. Cao, RSC Adv., 2015, 5, 36656–36664 RSC.
- S. M. Abd el Haleem and B. G. Ateya, J. Electroanal. Chem. Interfacial Electrochem., 1981, 117, 309–319 CrossRef CAS.
- J. Huang, P. Xu, D. Cao, X. Zhou, S. Yang, Y. Li and G. Wang, J. Power Sources, 2014, 246, 371–376 CrossRef CAS.
- W. Liu, C. Ju, D. Jiang, L. Xu, H. Mao and K. Wang, Electrochim. Acta, 2014, 143, 135–142 CrossRef CAS.
- M.-J. Deng, C.-Z. Song, P.-J. Ho, C.-C. Wang, J.-M. Chen and K.-T. Lu, Phys. Chem. Chem. Phys., 2013, 15, 7479–7483 RSC.
- M. Huang, X. L. Zhao, F. Li, L. L. Zhang and Y. X. Zhang, J. Power Sources, 2015, 277, 36–43 CrossRef CAS.
- F. Cao, G. X. Pan, P. S. Tang and H. F. Chen, J. Power Sources, 2012, 216, 395–399 CrossRef CAS.
- X. Yan, X. Tong, J. Wang, C. Gong, M. Zhang and L. Liang, J. Alloys Compd., 2014, 593, 184–189 CrossRef CAS.
- S. E. Moosavifard, M. F. El-Kady, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, ACS Appl. Mater. Interfaces, 2015, 7, 4851–4860 CAS.
- Z. Lin, X. Yan, J. Lang, R. Wang and L.-B. Kong, J. Power Sources, 2015, 279, 358–364 CrossRef CAS.
- W. Liu, X. Li, M. Zhu and X. He, J. Power Sources, 2015, 282, 179–186 CrossRef CAS.
- V. Senthilkumar, Y. S. Kim, S. Chandrasekaran, B. Rajagopalan, E. J. Kim and J. S. Chung, RSC Adv., 2015, 5, 20545–20553 RSC.
- D. P. Dubal, N. R. Chodankar, G. S. Gund, R. Holze, C. D. Lokhande and P. Gomez-Romero, Energy Technol., 2015, 3, 168–176 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



