Fabrication of hollow-structured composite microspheres with amphiphilic and superparamagnetic properties
Xiaohui Zhan†
b,
Liqin Xie†*a,
Hongli Chena,
Yao Wu*b and
Zhongwei Gub
aCollege of Life Science and Technology, Xinxiang Medical University, 601 Jinsui Road, Xinxiang 453003, China. E-mail: xieliqin230@163.com
bNational Engineering Research Center for Biomaterials, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: wuyao@scu.edu.cn
First published on 15th January 2016
Abstract
Solvent etching is a general method for the fabrication of hollow-structured nano/micro-spheres with multiple functions. In this study, we report a facile route for the preparation of hollow composite microspheres, which are amphiphilic and superparamagnetic. In a template of magnetic composite microspheres, P(St-AA)/Fe3O4/PAA, P(St-AA) was selectively etched, resulting in a hydrophobic hollow structure with a shell of hydrophilic polymeric brushes. The effects of etching on the morphology, hollow structure, saturation magnetization, amphiphilic property and cytocompatibility were investigated. Characterization showed that the hollow composite microspheres obtained possessed a well-defined spherical structure, a high saturation magnetization, amphiphilic properties and good cytocompatibility, making them promising as a potential biomaterial in magnetic resonance imaging and drug delivery systems.
1 Introduction
Recently, the design and preparation of superparamagnetic nano/micro-spheres with a hollow structure and tailored functions have attracted considerable attention because of their extensive application in biomedical fields such as magnetic resonance imaging, drug delivery, biocatalysts and nanoreactors.1–5A common strategy for fabricating hollow-structured superparamagnetic multifunctional nano/micro-spheres is a combination of layer-by-layer (LBL) assembly with etching.6–10 LBL assembly is a popular technique due to its mild preparation conditions and ability to introduce tailored functions. However, microspheres prepared by the LBL technique showed poor stability. This was because LBL assembly11,12 employed electrostatic interactions as the driving force and these were too sensitive to pH and ionic strength.13 For constructing hollow structures, the etching method has attracted significant interest as a simple, highly versatile approach.14 Etching modes include hydrothermal etching,6 dialysis,7–10 ion etching,15 solvent etching16–18 and calcination.19–21 Among all the etching methods, solvent etching offers moderate reaction conditions and a facile operation. Based on solubility differences, templates were selectively removed by means of an etching solvent. For example, hydrofluoric acid was usually used to etch silica from a composite material22 and organic solvents were employed to separate polymers from inorganic substrates.17 However, in the abovementioned studies, the templates were just the substrates for a subsequent modification and these were completely removed from a system.
In this work, we introduce a type of superparamagnetic composite microsphere prepared via a LBL technique. However, the superparamagnetic nanoparticles and functional polymers were assembled via coordinate-covalent bonds rather than electrostatic interactions. In the etching process, the polymeric template was partly removed, resulting in a hollow structure with amphiphilic properties. The hollow-structured magnetic microspheres obtained are stable and monodispersed in aqueous or organic solvents; the fabricating process is illustrated in Scheme 1. Poly(styrene-co-acrylic acid) (P(St-AA)), poly(styrene-co-acrylic acid)/Fe3O4 (P(St-AA)/Fe3O4) and poly(styrene-co-acrylic acid)/Fe3O4/polyacrylic acid (P(St-AA)/Fe3O4/PAA) were prepared in our previous work.23,24 In order to obtain multifunctional composite microspheres, solvent etching was employed for fabricating hollow, amphiphilic superparamagnetic composite microspheres and the effects of etching on the morphology, hollow structure, saturation magnetization, amphiphilic properties and cytocompatibility were investigated. Owing to their superparamagnetism, amphiphilicity, hollow structure and cytocompatibility, the multifunctional microspheres could be used in magnetic resonance imaging and drug delivery for achieving integration of tracing and chemotherapy.25–28
2 Materials and methods
2.1 Materials
P(St-AA), P(St-AA)/Fe3O4 and P(St-AA)/Fe3O4/PAA were synthesized in our previous work. Tetrahydrofuran (THF), N,N-dimethylformamide (DMF), toluene and ethanol were provided by Chengdu Kelong Co. (Chengdu, China). 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt (WST-8), penicillin and streptomycin were purchased from Sigma-Aldrich (St Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone (Logan, UT, USA). Mouse fibroblast cells (3T3) and human hepatic carcinoma cells (HepG2) were obtained from the Chinese Academy of Science Cell Bank for Type Culture Collection (Shanghai, China). All the reagents were of analytical grade and were used as received. Milli-Q water was used throughout the experiments.2.2 Measurements and characterization
Thermogravimetric analysis (TGA) measurements were performed with simultaneous thermal analysis (STA 449 C Jupiter, NETZSCH). The mass loss of the dried samples was monitored under nitrogen at temperatures ranging from 35 to 800 °C with a heating rate of 10 K min−1. The magnetization of the dried samples was measured using a vibrating sample magnetometer (VSM, Model BHV-525, Riken Japanese Electronics Company) with a field varying from 0 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe at 300 K. Scanning electron microscopy (SEM, S-4800, Japan electronic) and transmission electron microscopy (TEM, JEM-2100, Japan electronic) measurements were carried out with samples prepared on a single monocrystalline silicon sheet in air under ambient conditions. Hydrodynamic diameters and the polydispersity index (PDI) of the particles were measured by dynamic light scattering (DLS, Malvern Nano-ZS, λ = 632.8 nm). Samples were dispersed in a related solvent in a glass cuvette and tested at 25 °C. The surface wettability was measured at ambient temperature on a Krüss optical contact angle measuring instrument, DSA100 (Germany), the measurement range of which is 0–180° and the angle precision ±0.1°. The sample was coated uniformly onto the silicon wafer by spin-coating. Once a water or organic solvent droplet had been dropped onto the surface of the sample film, the machine began to take photos.
000 Oe at 300 K. Scanning electron microscopy (SEM, S-4800, Japan electronic) and transmission electron microscopy (TEM, JEM-2100, Japan electronic) measurements were carried out with samples prepared on a single monocrystalline silicon sheet in air under ambient conditions. Hydrodynamic diameters and the polydispersity index (PDI) of the particles were measured by dynamic light scattering (DLS, Malvern Nano-ZS, λ = 632.8 nm). Samples were dispersed in a related solvent in a glass cuvette and tested at 25 °C. The surface wettability was measured at ambient temperature on a Krüss optical contact angle measuring instrument, DSA100 (Germany), the measurement range of which is 0–180° and the angle precision ±0.1°. The sample was coated uniformly onto the silicon wafer by spin-coating. Once a water or organic solvent droplet had been dropped onto the surface of the sample film, the machine began to take photos.
2.3 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The precipitates were then redispersed in a related mixed solution of H2O/THF or H2O/DMF (V1/V2 = 1
000 rpm. The precipitates were then redispersed in a related mixed solution of H2O/THF or H2O/DMF (V1/V2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and centrifuged for three cycles.
9) and centrifuged for three cycles.Additionally, P(St-AA) microspheres (10 mg) could be dispersed in 1 mL ethanol with ultrasonic vibration. The dispersion was then added into 9 mL toluene with stirring for 24 h at room temperature. The following steps were as described above. The related mixed solution was toluene/ethanol (V1/V2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9).
9).
The product obtained was the hollow-structured microspheres, h-P(St-AA), which were then re-dispersed in distilled water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9)). The hollow composite microspheres, h-P(St-AA)/Fe3O4, were re-dispersed in distilled water for further characterization.
9)). The hollow composite microspheres, h-P(St-AA)/Fe3O4, were re-dispersed in distilled water for further characterization.As previously mentioned, P(St-AA)/Fe3O4/PAA (10 mg) was dispersed in a mixed solvent (10 mL) of H2O/THF, H2O/DMF or ethanol/toluene (V1/V2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and the mixture was stirred for 24 h at room temperature. The etched P(St-AA)/Fe3O4/PAA composite microspheres were collected by magnetic separation and washed three times with a related solution. The hollow composite microspheres obtained, h-P(St-AA)/Fe3O4/PAA, were re-dispersed in distilled water for the further characterization.
9) and the mixture was stirred for 24 h at room temperature. The etched P(St-AA)/Fe3O4/PAA composite microspheres were collected by magnetic separation and washed three times with a related solution. The hollow composite microspheres obtained, h-P(St-AA)/Fe3O4/PAA, were re-dispersed in distilled water for the further characterization.
In vitro cytotoxicity was evaluated by performing 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt (WST-8) assays on 3T3 and HepG2 cell lines. The cells, which were seeded in a 96-well cell culture plate at a density of 2000 cells per well, were cultured in a humidified incubator under 5% CO2 at 37 °C for 24 h. Afterwards, 3T3 and HepG2 cells were treated with serial dilutions of h-P(St-AA)/Fe3O4/PAA in DMEM (100 mL per well) and further incubated for 24 h. After removing the media, 100 mL DMEM containing 10 mL WST-8 was added to each well and incubated at 37 °C for an additional 2 h. Finally, the absorbance at 450 nm of each well was monitored using a microplate reader (Thermo Fisher Scientific Varioskan Flash) to determine the relative cell viability.
3 Results and discussions
3.1 Surface morphology characterization
Before and after solvent etching, the morphologies of the P(St-AA) microspheres were observed by scanning electron microscopy (SEM), as shown in Fig. 1. Before solvent etching, the P(St-AA) microspheres, which had a uniform diameter and a smooth surface, were monodispersed in distilled water (Fig. 1a). The hydrodynamic diameter was 237 ± 3 nm with a very narrow polydispersity index (PDI) of 0.051. After etching in H2O/THF, the degree of swelling of each P(St-AA) microsphere was different. In Fig. 1b, the broken P(St-AA) microspheres indicated a hollow structure. Moreover, there were some nanospheres that were about 100 nm in diameter. These might be segments of polystyrene, which dissolved from the P(St-AA) microspheres and formed nanospheres during mechanical stirring in H2O/THF solution. Although the P(St-AA) microspheres swelled to a larger size, they were still monodispersed in the solution of H2O/THF and appeared to have a smooth surface. The results from dynamic light scattering (DLS) showed that the hydrodynamic diameter was about 360 nm and the PDI was 0.222. The DLS results were consistent with the SEM images; the etched P(St-AA) microspheres swelled to form nonuniform particles. In Fig. 1c, the P(St-AA) microspheres were etched by H2O/DMF. There was significant adhesion among the P(St-AA) microspheres. This might be because segments of polyacrylic acid were coated on the outer layer of the P(St-AA) microspheres, which were more easily dissolved in the polar solution of H2O/DMF. However, many nanospheres (about 100 nm in diameter) could be distinguished in this image. DLS results showed that the hydrodynamic diameter was about 98 nm and the PDI was 0.115. These nanospheres may be segments of polystyrene that re-formed into a spherical shape under mechanical stirring in H2O/DMF solution. In Fig. 1d, P(St-AA) microspheres almost dissolved to form a polymeric membrane; several swollen P(St-AA) microspheres formed a spherical structure. This illustrated that the polystyrene, as the framework of the P(St-AA) microspheres, dissolved well in a weak polar solution of ethanol/toluene. Although the segments of polyacrylic acid were poorly dissolved in ethanol/toluene, these segments were too soft to construct a spherical structure at room temperature. DLS results showed that the hydrodynamic diameter of the swollen P(St-AA) microspheres was about 247 nm with a wide PDI of 0.493. Under the same volume ratio, the order of polarity of the solutions is H2O/DMF > H2O/THF > ethanol/toluene. According to the structural properties of the polymeric segments and the polarity of the solutions, it could be deduced that polystyrene dissolved easily in ethanol/toluene just as polyacrylic acid dissolved easily in H2O/DMF. As a result, the P(St-AA) microspheres were etched into hollow structures and maintained a well-defined spherical morphology only in a solution of H2O/THF. | ||
| Fig. 1 The P(St-AA) microspheres were dispersed in distilled water (a) and the etched P(St-AA) microspheres in H2O/THF (b), H2O/DMF (c) or ethanol/toluene (d) mixed solutions. | ||
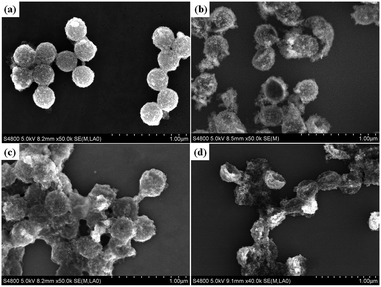
As the Fe3O4 nanoparticles were anchored onto the P(St-AA) microspheres, the surface of the composite microspheres, P(St-AA)/Fe3O4, became rougher, as shown in Fig. 2a. Before etching, the hydrodynamic diameter of P(St-AA)/Fe3O4 was about 266 nm (PDI = 0.191). After etching by H2O/THF (Fig. 2b), the diameter of the swollen P(St-AA)/Fe3O4 composite microspheres was enlarged slightly. The etched P(St-AA)/Fe3O4 composite microspheres appeared to have an irregular spherical shape and possessed poorer monodispersity. The DLS results also showed that the hydrodynamic diameter was 419 nm with a wide PDI of 0.57. We rationalized that the etched P(St-AA) microspheres were slightly broken and that the anchored Fe3O4 nanoparticles dropped off the P(St-AA)/Fe3O4 composite microspheres. Subsequently, the shed Fe3O4 nanoparticles aggregated with themselves or with the broken P(St-AA)/Fe3O4 composite microspheres, which resulted in irregular spherical shapes and poorer monodispersity. In Fig. 2c, the outer layer of the P(St-AA), which combined with Fe3O4 nanoparticles, was dissolved in the polar solution of H2O/DMF. As a result, there was significant adhesion of P(St-AA)/Fe3O4 composite microspheres and aggregation of the shed Fe3O4 nanoparticles. As indicated by the DLS results, the hydrodynamic diameter was 421 nm with a wide PDI of 0.591. In Fig. 2d, the P(St-AA)/Fe3O4 composite microspheres still retained a spherical structure after etching in ethanol/toluene. As illustrated in the last paragraph, the polystyrene was easily dissolved in ethanol/toluene. As the outer layer of the P(St-AA) microspheres, the segments of polyacrylic acid were poorly dissolved in ethanol/toluene. Due to combination of the polyacrylic acid layer with the Fe3O4 nanoparticles, the combined layer was fairly solid and thereby served as a support for constructing the spherical framework. However, the DLS results showed that the PDI was much wider than 0.5, therefore the hydrodynamic diameter characterized by DLS cannot be referenced.
As the PAA brushes were grafted to the P(St-AA)/Fe3O4 composite microspheres, the hydrodynamic diameter was enlarged to 327 nm with a PDI of 0.135. The P(St-AA)/Fe3O4/PAA composite microspheres possessed a well-defined spherical structure and good monodispersity, as shown in Fig. 3a. Through etching in H2O/THF, the hollow structure was shown in the broken P(St-AA)/Fe3O4/PAA composite microspheres (Fig. 3b). The shed Fe3O4 nanoparticles were partly aggregated. Because of the irregular structure, the hydrodynamic diameter was enlarged to 355 nm and the PDI correspondingly increased to 0.224. When the etching solution was replaced by H2O/DMF, the etched P(St-AA)/Fe3O4/PAA composite microspheres maintained their unbroken spherical structure (Fig. 3c). Under the same etching conditions, comparing with the etched P(St-AA)/Fe3O4 composite microspheres, the etched P(St-AA)/Fe3O4/PAA composite microspheres showed good monodispersity. This demonstrated that the grafted PAA brushes prevented adhesion of the composite microspheres and aggregation of the Fe3O4 nanoparticles. The corresponding hydrodynamic diameter was about 296 nm with a PDI of 0.166. In Fig. 3d, the etched P(St-AA)/Fe3O4/PAA composite microspheres showed a hollow spherical structure. Under the same etching conditions, compared with the etched P(St-AA)/Fe3O4 composite microspheres, P(St-AA)/Fe3O4/PAA composite microspheres possessed a relatively regular spherical framework and an obvious hollow structure. This was because both the outer layer of P(St-AA) and the PAA brushes were coordinated with the Fe3O4 nanoparticles, forming a soft–hard–soft sandwich structure, which was more suitable for constructing a spherical framework. Even so, the PDI was so wide that the hydrodynamic diameter cannot be referenced.
In addition, TEM was employed to observe the hollow structure. The morphologies of h-P(St-AA)/Fe3O4/PAA composite microspheres etched in H2O/THF solution are shown in Fig. 4. The wall of the hollow composite microspheres was comparatively thick, as shown in the SEM image in Fig. 4b, which explained why the etched composite microspheres could maintain their spherical structure. In Fig. 4c, the TEM image indicated that the etched composite microspheres had an irregular spherical structure and that the edge of the microspheres was a deep color. The irregular spherical structure was generated by solvent etching. The deep color at the edge demonstrated that the etched composite microspheres had a hollow structure, even though they were unbroken. The morphologies of the etched composite microspheres are shown more clearly in Fig. 4d, where the etched composite microsphere has a well-defined spherical structure and the deep color is regularly distributed over the edge of the sphere.
 | ||
| Fig. 4 SEM (a and b) and TEM (c and d) images of P(St-AA)/Fe3O4/PAA composite microspheres after etching with H2O/THF solution. | ||
3.2 Magnetic content and saturation magnetization
Due to the etching effect of H2O/THF solution, hollow structures were generated in the P(St-AA)/Fe3O4 and P(St-AA)/Fe3O4/PAA composite microspheres, resulting in an increase in magnetic content and saturation magnetization. Thermogravimetric analysis (TGA) measurement and a vibrating sample magnetometer were employed for characterizing the magnetic content and the saturation magnetization, respectively. The TGA curves and magnetization curves are shown in Fig. 5. For the TGA curves, the weight loss at about 360 °C was ascribed to the decomposition of the free organic component. The polymers bonded to Fe3O4 nanoparticles, such as partial P(St-AA) chains or PAA brushes, were desorbed and decomposed at a relatively higher temperature of 680 °C.29 The magnetic content of the P(St-AA)/Fe3O4 composite microspheres increased from 47.8% (line a) to 54.7% (line b) because of the decrease in the mass of the hollow. Under the same conditions, the magnetic content of the P(St-AA)/Fe3O4/PAA composite microspheres increased from 43.3% (line c) to 46.1% (line d). For the magnetization curves, the change trends were similar to those of magnetic curves. The saturation magnetization of P(St-AA)/Fe3O4 composite microspheres increased from 36.99 emu g−1 (line a) to 39.03 emu g−1 (line b) and that of P(St-AA)/Fe3O4/PAA composite microspheres increased from 27.81 emu g−1 (line c) to 32.18 emu g−1 (line d). These etched composite microspheres could be collected more quickly under an external magnet due to the increase in the magnetic content and saturation magnetization. The magnetic hysteresis loop coincided well, which showed that the etched composite microspheres still maintained their superparamagnetic properties.3.3 Amphiphilic properties
To identify the amphiphilic properties, contact angles of liquid with the materials were characterized to determine the surface wettability. The smaller the angle, the better the surface wettability. Firstly, the magnetic microspheres of h-P(St-AA)/Fe3O4 and h-P(St-AA)/Fe3O4/PAA were coated onto a silicon wafer by spin-coating. Water or toluene was dropped onto the surface of the magnetic microsphere film. As shown in Fig. 6, the contact angle of water on P(St-AA)/Fe3O4 was 36.7°, which showed good hydrophilic properties. The contact angle of toluene on P(St-AA)/Fe3O4 was 53.5°, which showed the relatively good hydrophobic properties. These results proved that P(St-AA)/Fe3O4 microspheres possessed both hydrophilicity and hydrophobicity. After the PAA-brushes were grafted to the P(St-AA)/Fe3O4 microspheres, the etched magnetic microspheres of h-P(St-AA)/Fe3O4/PAA showed better hydrophilicity and hydrophobicity. The contact angles for h-P(St-AA)/Fe3O4/PAA with water or toluene were 29.8° and 44.5°, respectively. These results demonstrated the hydrophobic hollow and hydrophilic shell structure.To identify the amphiphilic properties at the macroscopic level, the etched magnetic composite microspheres were dispersed in a single organic solvent: THF, DMF or toluene. Fig. 7 shows that h-P(St-AA)/Fe3O4 composite microspheres were dispersed homogeneously in a single organic solvent. Also, h-P(St-AA)/Fe3O4/PAA composite microspheres were well dispersed in THF and DMF. In toluene, h-P(St-AA)/Fe3O4/PAA composite microspheres were well dispersed ultrasonically but quickly aggregated and precipitated without an external magnetic field. This was because PAA brushes were not well dissolved in toluene, resulting in the aggregation and precipitation of the h-P(St-AA)/Fe3O4/PAA composite microspheres. Summarily, these etched magnetic composite microspheres showed good dispersibility in a single organic solvent or in water, which illustrated their amphiphilic properties.
3.4 Cytocompatibility
The hollow-structured and amphiphilic magnetic microspheres of h-P(St-AA)/Fe3O4/PAA are potential biomaterials for magnetic resonance imaging and drug delivery systems. It is critical that these hollow magnetic microspheres possess low cytotoxicity. Normal cells of mouse fibroblast 3T3 and tumor cells of human hepatic carcinoma HepG2 were incubated with h-P(St-AA)/Fe3O4/PAA for 24 h. Control experiments were carried out without magnetic microspheres and the cell viability was determined as 100%. Fig. 8 shows the viability of the two types of cells. The concentrations of h-P(St-AA)/Fe3O4/PAA were 10, 100, 200, 300 and 400 μg mL−1. When the concentration of h-P(St-AA)/Fe3O4/PAA was 10 μg mL−1, the cell viabilities of 3T3 and HepG2 were above 90%. As the concentration of h-P(St-AA)/Fe3O4/PAA increased, the cell viabilities decreased. When the concentration of h-P(St-AA)/Fe3O4/PAA was 400 μg mL−1, the cell viabilities of 3T3 and HepG2 were 77% and 83%, respectively. Thus, it can be concluded that h-P(St-AA)/Fe3O4/PAA has very low cytotoxicity towards both normal cells (3T3) and tumor cells (HepG2). These results illustrated that h-P(St-AA)/Fe3O4/PAA possessed good cytocompatibility. | ||
| Fig. 8 Viability of 3T3 and HepG2 cells incubated with different concentrations of h-P(St-AA)/Fe3O4/PAA magnetic microspheres for 24 h. | ||
4 Conclusions
In this study, we synthesized a type of hollow-structured composite microsphere with amphiphilic and superparamagnetic properties via solvent etching. THF, DMF and toluene were selected as the etching solvents because of their miscibility with water and ethanol. Meanwhile, they showed different dissolving capacities with respect to polymers. This method provided a simple and mild route for constructing multifunctional hollow-structured composite materials. The resulting materials showed good amphiphilic properties, hollow structures and a fast magnetic response, as well as good cytocompatibility, which implied that they could be applied as magnetic resonance imaging contrast agents and drug delivery carriers for tracing and chemotherapy.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 51503175, 51273127, 21571134, U1304819), Project in the Science and Technology Support Program of Sichuan Province (No. 2014GZ0049), Doctor's Initial Funding (No. 505089) and Cultivation Funding (No. 2014QN139) of Xinxiang Medical University.Notes and references
- C. F. Lee, M. L. Hsu, C. H. Chu and T. Y. Wu, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 3441 CrossRef CAS.
- X. Kang, Z. Cheng, D. Yang, P. Ma, M. Shang, C. Peng, Y. Dai and J. Lin, Adv. Funct. Mater., 2012, 22, 1470 CrossRef CAS.
- P. L. Moises, B. Vaz, V. Salgueirino and M. A. Correa-Duarte, Chem.–Eur. J., 2013, 19, 12196 CrossRef PubMed.
- Y. Li and J. Shi, Adv. Mater., 2014, 26, 3176 CrossRef CAS PubMed.
- Q. Zhang, I. Lee, J. Ge, F. Zaera and Y. Yin, Adv. Funct. Mater., 2010, 20, 2201 CrossRef CAS.
- W. Li, Y. Deng, Z. Wu, X. Qian, J. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Zhao, J. Am. Chem. Soc., 2011, 133, 15830 CrossRef CAS PubMed.
- P. Du, J. Zeng, B. Mu and P. Liu, Mol. Pharmaceutics, 2013, 10, 1705 CrossRef CAS PubMed.
- B. Mu, P. Liu, X. Li, P. Du, Y. Dong and Y. Wang, Mol. Pharmaceutics, 2012, 9, 91 CrossRef CAS PubMed.
- X. Zhao and P. Liu, Mol. Pharmaceutics, 2014, 11, 1599 CrossRef CAS PubMed.
- X. Zhao, P. Du and P. Liu, Mol. Pharmaceutics, 2012, 9, 3330 CrossRef CAS PubMed.
- O. S. Sakr and G. Borchard, Biomacromolecules, 2013, 14, 2117 CrossRef CAS PubMed.
- Y. Wang, A. S. Angelatos and F. Caruso, Chem. Mater., 2008, 20, 848 CrossRef CAS.
- P. Liu, X. Li, B. Mu, P. Du, X. Zhao and Z. Zhong, Ind. Eng. Chem. Res., 2012, 51, 13875 CrossRef CAS.
- Y. Chen, H. Chen, L. Guo, Q. He, F. Chen, J. Zhou, J. Feng and J. Shi, ACS Nano, 2010, 4, 529 CrossRef CAS PubMed.
- D. G. Choi, H. K. Yu, S. G. Jang and S. M. Yang, J. Am. Chem. Soc., 2004, 126, 7019 CrossRef CAS PubMed.
- M. Zhang, Y. Lan, D. Wang, R. Yan, S. Wang, L. Yang and W. Zhang, Macromolecules, 2011, 44, 842 CrossRef CAS.
- C. Yang and P. Liu, Ind. Eng. Chem. Res., 2012, 51, 13346 CrossRef CAS.
- G. Li, Q. Shi, S. J. Yuan, K. G. Neoh, E. T. Kang and X. Yang, Chem. Mater., 2010, 22, 1309 CrossRef CAS.
- W. F. Ma, C. Zhang, Y. T. Zhang, M. Yu, J. Guo, Y. Zhang, H. J. Lu and C. C. Wang, Langmuir, 2014, 30, 6602 CrossRef CAS PubMed.
- H. K. Lee, D. Sakemi, R. Selyanchyn, C. G. Lee and S. W. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 57 CAS.
- F. He, P. Yang, D. Wang, C. Li, N. Niu, S. Gai and M. Zhang, Langmuir, 2011, 27, 5616 CrossRef CAS PubMed.
- G. Li, G. Liu, E. T. Kang, K. G. Neoh and X. Yang, Langmuir, 2008, 24, 9050 CrossRef CAS PubMed.
- L. Xie, S. Ma, Q. Yang, F. Lan, Y. Wu and Z. Gu, RSC Adv., 2014, 4, 1055 RSC.
- L. Xie, F. Lan, W. Li, Z. Liu, S. Ma, Y. Wu and Z. Gu, Colloids Surf., B, 2014, 123, 413 CrossRef CAS PubMed.
- X. Li, H. Li, G. Liu, Z. Deng, S. Wu, P. Li, Z. Xu, H. Xu and P. K. Chu, Biomaterials, 2012, 33, 3013 CrossRef CAS PubMed.
- P. Du, J. Zeng, B. Mu and P. Liu, Mol. Pharmaceutics, 2013, 10, 1705 CrossRef CAS PubMed.
- S. W. Cao, Y. J. Zhu, M. Y. Ma, L. Li and L. Zhang, J. Phys. Chem. C, 2008, 112, 1851 CAS.
- S. J. Park, H. S. Lim, Y. M. Lee and K. D. Suh, RSC Adv., 2015, 5, 10081 RSC.
- Q. Lan, C. Liu, F. Yang, S. Y. Liu, J. Xu and D. J. Sun, J. Colloid Interface Sci., 2007, 310, 260 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |