The prediction of the optimum compositions of a parenteral nanoemulsion system loaded with a low water solubility drug for the treatment of schizophrenia by artificial neural networks
Wan Sarah Samiun*,
Mahiran Basri*,
Hamid Reza Fard Masoumi and
Nurshafira Khairudin
Nanodelivery Group, Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia. E-mail: mahiran@upm.edu.my; wansarahsamiun@yahoo.com
First published on 18th January 2016
Abstract
Aripiprazole was encapsulated in a palm kernel oil esters nanoemulsion for the purpose of brain delivery via intravenous administration. High shear and high pressure homogenizers were applied for formulating the low water solubility drug in the nanoemulsion system, which was stabilized using different emulsifiers; lecithin, Tween 80 and glycerol. The artificial neural networks (ANNs) modeling of the nanoemulsion formulation was carried out to achieve the minimum particle size. The effects of the amount of palm kernel oil ester (PKOE) (3–6%, w/w), lecithin (2–3%, w/w), Tween 80 (0.5–1%, w/w), glycerol (1.5–3%, w/w), and water (87–93%, w/w) on the particle size were considered as inputs to the network. The particle size of the samples with various compositions was measured as an output. To obtain the optimum topologies, the ANNs were trained using Incremental Back Propagation (IBP), Genetic Algorithm (GA), Batch Back Propagation (BBP), Quick Propagation (QP), and Levenberg–Marquardt (LM) algorithms for the testing data set. The topologies were determined by the indicator of the minimized root mean squared error (RMSE) for each algorithm. According to the results, QP-5-4-1, GA-5-12-1, IBP-5-11-1, BBP-5-10-1, and LM-5-9-1 were selected as the optimized topologies. It was found that the optimal algorithm and topology were the quick propagation and the configuration with 5 input, 4 hidden and 1 output nodes. Conclusively, ANN models were developed for the prediction of the particle size of nanoemulsions loaded with aripiprazole and its stable nanoemulsion system, which could be used effectively for intravenous administration.
Introduction
Schizophrenia is a serious, chronic and debilitating mental illness for which most patients require long term treatment with antipsychotic treatment.1 This mental illness is characterized by positive symptoms (e.g., hallucinations, delusions and deranged thoughts), negative symptoms (e.g., loss of motivation, restricted emotional experience, poverty of speech) and cognitive impairment. The first hypothesis formulated that could explain the pathophysiology of this mental illness, is namely the “dopamine theory”, which attributed a prominent role to dopamine system dysregulation. In recent years, a medication (aripiprazole) has received a growing attention towards the treatment of schizophrenia.2–5Schizophrenia disease is mostly related to the Central Nervous System (CNS) in the body. All organisms with a well-developed CNS have a blood–brain barrier (BBB).6 The BBB acts as a barrier to prevent any macromolecules from entering the brain.7
Aripiprazole is regarded as a third generation antipsychotic drug (APD) with excellent therapeutic efficacy in controlling the symptoms of schizophrenia, a low incidence of extra-pyramidal side effects (EPS) and weight gain side effects.8–11
Aripiprazole possesses poorly-water soluble characteristics in the form of its salt. To ensure that it can reach the target cell effectively, a higher dosage of aripiprazole is needed. However, in some clinical cases, a higher dosage of aripiprazole can cause some side effects.12 A nanoemulsion-based aripiprazole carrier could improve the solubility of the drug in the dispersed phase, drug penetration through the blood–brain barrier (BBB) and target cells due to its extremely small size. Thus, a smaller dosage of aripiprazole is preferred to reduce its side effects. For enhancing the poorly-water soluble molecule's dissolution rate, the surface area is purposely increased by decreasing the particle size of the drug molecule. The performance can extremely affected by increasing the surface area.13 In this context, the development of new drug nanodelivery systems to increase drug bioavailability and reduce adverse effects has been claimed as a good option.
An emulsion consists of a mixture of two immiscible liquids, which are dispersed throughout each other. Water and oils is considered as its basic components, which requires a surfactant to decrease the interfacial tension and maintain the stability of the emulsion. An emulsion can be prepared in the form of oil-in-water or water-in-oil.14 Palm kernel oil esters (PKOEs) consist of a higher amount of short chain esters when compared to other oils. In the pharmaceutical industry, palm kernel oil esters are used in formulations because they can be a good carrier for active components. Due to its lower slip melting point and higher saponification value, palm kernel oil esters have unique properties for nanoemulsions and can be used in many applications such as cosmetics15 and pharmaceuticals.16
A nanoemulsion is thermodynamically stable transparent (or translucent) system of oil, water, and surfactants, having a droplet size usually in the range of 20–200 nm.17,18 It has been proven that a very small particle size of emulsion can provide effective encapsulation for delivery in the human body.19 Nanoemulsions present good drug delivery and parenteral delivery due to their small nanometer size particles, biocompatibility, relative stability, ability to solubilize high quantities of hydrophobic compounds, ability to reduce the toxicity of drugs, and ability to protect drugs from hydrolysis and enzymatic degradation under physiological conditions.20
Nanoemulsions are classified as non-equilibrium systems. To form the nanoemulsion, energy input from a mechanical device or from the chemical potential of the components is needed.21 There are two emulsification methods that have been used to make nanoemulsions. The first emulsification method is called the dispersion or high energy emulsification method, which uses mechanical energy (such as high-pressure homogenization).22 This method is widely used in industry to produce small and uniform droplet size of emulsions. The second emulsification method is the low energy emulsification technique. This method consists of the stepwise addition of one component to a mixture of the other components at a constant temperature.23
In recent years, artificial neural networks (ANNs) were introduced in pharmaceutical applications as an effective tool to solve complex multivariate non-linear relationships.24,25 ANNs are artificial neurons used to simulate the method in which biological neurons process information.26 ANNs consist of an interconnected group of neurons in the modeling process, which are used to give predictions on the behavior of a given system, designing a new process and analyzing existing processes. Moreover, this process can be carried out in a short period computing with high potential for adaptive performance of adequate quality.27–30 In pharmaceutical research, ANNs have been successfully used in analysis and modeling applications such as forming the controlled release drug delivery systems, enhancing the understanding of the formation nanoemulsions and the evaluation of the stability of nanoemulsions.31
In this study, the optimization of the composition of nanoemulsions containing aripiprazole with respect to the amount of oil, lecithin, Tween 80, glycerol and water was carried out. The response, which is the particle size, was studied to find the best model in the ANNs.
Methodology
Materials
Palm kernel oil esters (PKOEs) were synthesized in our laboratory via the enzymatic transesterification of palm kernel oil and oleyl alcohol.15 Pure soy bean lecithin (Lipoid S75) was purchased from Lipoid GmbH, Ludwigshafen, Germany. Glycerol was purchased from JT Baker, USA. Polysorbate 80 (Tween 80) was obtained from Fluka, Sigma-Aldrich Chemie GmbH, Germany. Aripiprazole was purchased from Laboratory & Scientific Enterprise, Malaysia. Water was deionized using a Milli-Q filtration system, Milipore, USA.Determination of the solubility of aripiprazole in oil
The solubility of aripiprazole in the PKOEs was determined. Different amounts of drug were added into the oil containing lecithin (3%). The solutions were kept under moderate magnetic stirring for 24 h to reach equilibrium. The samples were then centrifuged at 4500 rpm for 15 min. The best amount of drug was observed to be 0.1% in the composition formulation.Preparation of the emulsion formulation using low shear rate emulsification
Emulsions were prepared via low shear rate stirring emulsification using an overhead stirrer (IKA® RW20 Digital, Nara, Japan) at 300–305 rpm. Aripiprazole (0.1%) was dissolved in the oil phase, which was comprised PKOEs (3.0–6.0%) containing lecithin (2.0–3.0%) as the surfactant. Tween 80 (0.5–1.0%) was then added into the oil phase as a co-surfactant after the aripiprazole was completely dissolved. The oil phase was added dropwise into an aqueous phase consisting of glycerol and was continuously stirred to form a coarse emulsion. The mixing of the emulsions was carried out with a shear rate of 300–305 rpm for 3 h.Preparation of the nanoemulsion using high shear and high pressure homogenization
The emulsion prepared using low shear rate emulsification was homogenized with a high shear homogenizer (Kinematic Switzerland) at high speed (3500 rpm) for 15 minutes. The samples were further homogenized using a high-pressure homogenizer 1000 psi for 14 cycles. The final products were placed into sample bottles.Stability study
The freshly prepared samples were placed in a container and then stored in the refrigerator at ±5 °C for nine months. To test the stability of the samples, the samples were centrifuged at 4500 rpm for 15 min. The samples were then observed to observe if there any precipitation had occurred.Particle size measurement
The particle size distribution was measured by a diffusion method using a dynamic light scattering (DLS) particle analyzer (Zetasizer Nano ZS, Malvern Instruments, Malvern, UK). The size distribution using the diffusion of scattered laser light by the particles was measured. The measurement was conducted using the Photon Correlation Spectroscopy (PCS) principle.32,33Transmission electron microscopy (TEM)
The particle size was also measured using transmission electron microscopy (Hitachi H7100, Japan). A formvar coated copper grid was placed on top of a drop of the sample and left at room temperature (25 ± 0.5 °C) for 5 minutes. The filled copper grid was stained for 2 minutes using 2% phosphotungstic acid and air dried prior to TEM analysis.Experimental design
The modeling and optimization of the nanoemulsions containing aripiprazole were carried out using NeuralPower software version 2.5.28,29 In a mixture design wherein the composition was the factor of interest, the levels cannot be chosen arbitrarily. All fractions of the components must sum to unity.34 As Table 1 shows, a total of 27 experimental points have been randomly divided into two data sets for training (20 experiments) and testing (4 experiments), and a validation set (3 experiments). The software facilitated the option of randomization. The training and testing data sets were used to compare and ensure the robustness of the network parameters, respectively. Moreover, the testing set was utilized to avoid over fitting by controlling errors.35| Run no. | PKOEs (%) | Lecithin (%) | Tween 80 (%) | Glycerol (%) | Water (%) | Particle size (nm) | |
|---|---|---|---|---|---|---|---|
| Actual | Predicted | ||||||
| Training set | |||||||
| 1 | 6.00 | 3.00 | 0.50 | 1.50 | 89.00 | 124.97 | 127.67 |
| 2 | 6.00 | 2.00 | 1.00 | 3.00 | 88.00 | 87.62 | 87.62 |
| 3 | 4.50 | 2.00 | 0.75 | 2.25 | 90.50 | 86.55 | 86.56 |
| 4 | 5.25 | 2.50 | 0.63 | 1.88 | 89.75 | 103.43 | 103.44 |
| 5 | 6.00 | 2.00 | 1.00 | 1.50 | 89.50 | 86.18 | 88.34 |
| 6 | 3.00 | 2.50 | 0.50 | 1.50 | 92.50 | 81.35 | 81.35 |
| 7 | 3.00 | 2.00 | 0.50 | 3.00 | 91.50 | 84.43 | 84.43 |
| 8 | 6.00 | 3.00 | 0.50 | 1.50 | 89.00 | 130.37 | 127.67 |
| 9 | 6.00 | 2.00 | 1.00 | 1.50 | 89.50 | 90.48 | 88.34 |
| 10 | 3.00 | 2.00 | 1.00 | 2.25 | 91.75 | 66.20 | 64.22 |
| 11 | 6.00 | 3.00 | 1.00 | 2.25 | 87.75 | 89.59 | 89.59 |
| 12 | 3.00 | 3.00 | 0.50 | 2.25 | 91.25 | 81.39 | 81.39 |
| 13 | 3.00 | 2.00 | 0.75 | 1.50 | 92.75 | 71.56 | 71.56 |
| 14 | 6.00 | 2.00 | 0.50 | 2.25 | 89.25 | 124.57 | 124.57 |
| 15 | 3.00 | 3.00 | 1.00 | 1.50 | 91.50 | 65.25 | 65.25 |
| 16 | 5.25 | 2.75 | 0.88 | 2.63 | 88.50 | 89.07 | 89.07 |
| 17 | 4.50 | 2.50 | 0.75 | 3.00 | 89.25 | 87.12 | 87.12 |
| 18 | 4.50 | 3.00 | 1.00 | 1.50 | 90.00 | 80.26 | 80.26 |
| 19 | 3.00 | 3.00 | 1.00 | 3.00 | 90.00 | 65.55 | 65.55 |
| 20 | 4.50 | 2.00 | 0.50 | 1.50 | 91.50 | 106.13 | 106.14 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Testing set | |||||||
| 1 | 5.50 | 2.50 | 0.50 | 1.50 | 89.25 | 120.40 | 121.78 |
| 2 | 4.00 | 2.50 | 1.00 | 1.50 | 90.00 | 72.66 | 73.74 |
| 3 | 4.50 | 2.00 | 0.75 | 2.50 | 91.00 | 92.53 | 87.02 |
| 4 | 4.25 | 2.75 | 0.60 | 2.10 | 90.25 | 92.64 | 93.78 |
The ANN description
Artificial Neural Networks (ANNs) are computer programs, which are used for the simulation of some roles in the human brain using dissimilar learning algorithms. ANNs possess the extraordinary information processing attributes of the human brain, such as non-linearity, high parallelism, robustness, fault and failure tolerance, learning, capability to manage imprecise and distorted information, and the ability to generalize. ANNs, are able to manage multiple independent and dependent variables simultaneously in one model.36 The effectiveness of the ANN model is shown when modeling non-linear relationships between dependent and independent variables using an approach that is similar to a ‘black box’.37 This is because the performance of the ANN model is not needed for the end user to have extended understanding. The ANN model can predict and formulate optimization capabilities and also be updated with new raw data. After the models are trained, the response to new experimental conditions can be predicted using the ANN model.31ANNs that contain input, hidden and output layers provide the mathematical free functionalization of a complicated practical process.38 The layers, which consist of several nodes, are connected by multilayer normal feed-forward or feed-back connection formula.39 The hidden layer could be more than one parallel layer; however, the single hidden layer is universally suggested. The connection is that the nodes of a particular layer are connected to the nodes of the next layer. The nodes are simple artificial neurons, which simulate the behavior of biological neural networks.40 The nodes in the input layer are qualified by sending data via special weights to the nodes of the hidden layer and then to the output layer.39,41 The qualification is carried out by associated weights during the learning process via well-known learning algorithms.
The learning process
In the learning process, the weights are calculated by the weighted summation of the received data from the former layer and transferred to the next layer.42 The number of hidden nodes is obtained by a trial and error training calculation, which is examined from one to n nodes. In the process, the output of the hidden nodes in turn, acts as input to final (output) layer's nodes, which undergo a similar or different transformation. The universal learning algorithms are QP, IBP, GA, BBP and LM whereas the multilayer is the nodes' connection type.43 The usual transfer function is the logarithmic sigmoid for both hidden and output layers that is bounded from (0–1).29,44 The sigmoid bounded area is used to normalize the input and output data that is provided by the software scaling. The scaled data are passed into the first layer, propagated to the hidden layer and finally meet the output layer of the network. Each node in the hidden layer or in the output layer firstly acts as a summing junction, which modifies the inputs from the previous layer using the equation as follows:
 | (1) |
 | (2) |
 | (3) |
 | (4) |
Results and discussion
The topologies of the algorithms
The hidden layers are in between the input and output layers providing a link between the input and output layers. The structure of the hidden layer was constructed by examining a series of topologies with varied node number from 1 to 15 for each algorithm, which were the QP, IBP, BBP, GA, and LM algorithms. To determine the minimum value of the RMSE as an error function, the model learning was performed for the testing data set. However, to obtain the best model, it was carried out using 10 repetitions for each node to avoid random correlations due to the random initialization of the weight.To determine the optimized topology for each algorithm, the training was performed using the same steps for the QP, IBP, BBP, GA and LM algorithms. The minimum value of the RMSE was chosen among the 10 learning repetition data for each node and plotted in graphs of the minimum value of RMSE versus the nodes of the algorithms' hidden layer, which are shown in Fig. 1.
 | ||
| Fig. 1 The selected RMSE vs. node number for the composition of the nanoemulsion containing an aripiprazole network's hidden layer for QP, IBP, BBP, GA and LM. | ||
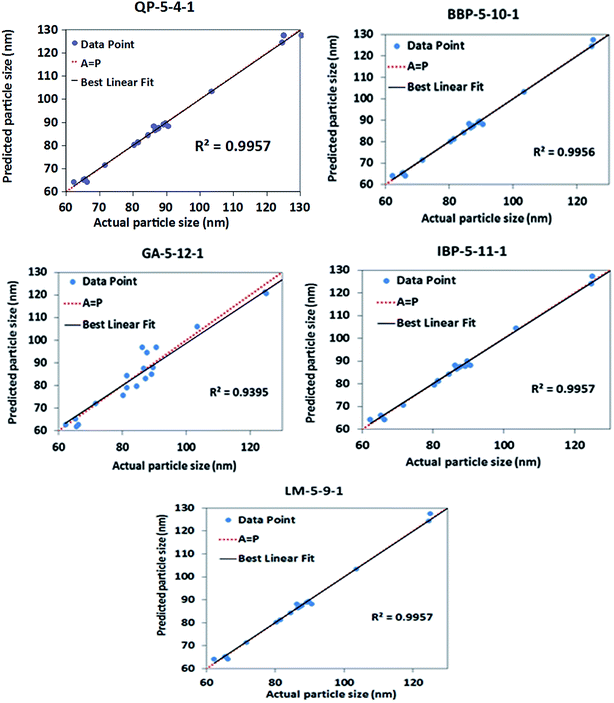
In a comparison of the best topology, one node of the 15 topologies in each algorithm was selected as the lowest RMSE. The selected topologies were 5-4-1, 5-11-1, 5-12-1, 5-10-1 and 5-9-1 for the QP, IBP, GA, BBP and LM algorithms, respectively. As shown in Fig. 2, the topology of QP-5-4-1 presented the lowest RMSE among the other topologies chosen as the provisional model for the nanoemulsion containing aripiprazole composition.
The model selection
To train the neural networks in the multivariate optimization of reaction, a gradient descent back-propagation algorithm in the QP version was applied. The experimental data of the mixed experimental design were divided into two sets: 20 of the data experiments were used as the training set, 4 of the data experiments were used as the test set and the remaining 3 data experiments used as the validation set (Table 1).The ANNs modeled the raw data and the best model created to provide better quality predictions for the particle size of the nanoemulsions. The RMSE, R2, and AAD of the model for the training and testing sets for the particle size are shown in Table 2. To select the final model for the particle size of the nanoemulsions, the values of RMSE, R2 and AAD were studied for the topologies of QP-5-4-1, BBP-5-10-1, IBP-5-11-1, GA-5-12-1 and LM-5-9-1. The best value of the RMSE was chosen based on 10 repeated runs. As shown in Table 2, QP had the lowest of the RMSE values, which was 2.614 in comparison with the other algorithms. Therefore, the performance of QP with the 5-4-1 topology was more effective than the IBP, BBP, GA and LM algorithms.
| Learning algorithms | Architecture | Training data | Testing data | ||||
|---|---|---|---|---|---|---|---|
| RMSE | R2 | AAD | RMSE | R2 | AAD | ||
| GA | 5-12-1 | 4.639 | 0.940 | 4.114 | 2.822 | 0.993 | 2.631 |
| LM | 5-9-1 | 1.228 | 0.996 | 0.728 | 2.801 | 0.982 | 2.350 |
| BBP | 5-10-1 | 1.244 | 0.996 | 0.744 | 2.712 | 0.979 | 2.182 |
| IBP | 5-11-1 | 1.327 | 0.996 | 1.095 | 2.706 | 0.984 | 2.203 |
| QP | 5-4-1 | 1.228 | 0.996 | 0.730 | 2.614 | 0.972 | 2.402 |
As the scatter plots depicted, in the comparison with the other topologies, the QP-5-4-1 topology presented an R2 value of 0.972, which was the best performance with a minimum RMSE. Then, the performed results of the topologies were used to calculate the RMSE, R2 and AAD. To calculate the R2 value, the prediction of the topologies and actual values of the particle size were plotted for the testing and training data sets, as shown in Fig. 2 and 3, respectively.
The QP-5-4-1 network
Fig. 4 shows a schematic of a multilayer perceptron feed-forward network of ANNs based on QP consisting of 5 inputs, one hidden layer with 4 nodes and one output. It illustrates the structure of the QP-5-4-1 topology as a final model for nanoemulsions containing aripiprazole. The input layer with 5 nodes (PKOEs, lecithin, Tween 80, glycerol and water) is the distributor for the hidden layer with 4 nodes, which were determined by the learning process. The input data of the hidden nodes were calculated by weighted summation (eqn (5)). Then, the output data of the hidden layer were transferred to the output layer (particle size) using a log-sigmoid function (eqn (6)).
 | (5) |
 | (6) |
Model verification
Table 3 shows that three random formulations were prepared to validate the final model. Verification of the model was carried out to examine the adequacy of the predicted particle size. The results indicated the good agreement between actual and predicted values.| Run no. | PKOEs (%) | Lecithin (%) | Tween 80 (%) | Glycerol (%) | Water (%) | Particle size (nm) | |
|---|---|---|---|---|---|---|---|
| Actual | Predicted | ||||||
| 1 | 3.75 | 2.00 | 0.75 | 2.50 | 91.00 | 76.73 | 78.76 |
| 2 | 4.25 | 2.25 | 0.50 | 2.25 | 90.75 | 96.18 | 98.49 |
| 3 | 4.50 | 2.50 | 0.60 | 2.10 | 90.30 | 98.04 | 96.13 |
Optimization of the compositions
Table 4 suggests the optimum formulation of nanoemulsions containing aripiprazole using the final ANN model. The smallest particle size (62.23 nm) was obtained using a nanoemulsion composition of 3% PKOEs, 2% lecithin, 1% Tween 80, 2.25% glycerol, and 91.75% water.| Methods | Optimal composition | Particle size (nm) | ||||||
|---|---|---|---|---|---|---|---|---|
| PKOEs (%) | Lecithin (%) | Tween 80 (%) | Glycerol (%) | Water (%) | Actual | Predicted | RSE% | |
| ANN (QP) | 3.00 | 2.00 | 1.00 | 2.25 | 91.75 | 62.23 | 64.38 | 3.34 |
Importance of the effective variables
Fig. 5 shows that the importance percentage of the input variables on the particle size of the nanoemulsions. The Tween 80 content at 29.45% is the most important factor controlling the particle size, followed by water at 19.34%, glycerol at 18.57%, lecithin at 17.39%, and PKOE at 15.25%. The presence of Tween 80 appeared to be most influential on the particle size. On the other hand, the effects of other variables such as water, glycerol, lecithin, and palm kernel oil esters were very strong on the particle size. As a result, none of the variables is neglectable in this study. | ||
| Fig. 5 The relative importance of the particle size of the nanoemulsion containing aripiprazole input variables of Tween 80, water, glycerol, lecithin and PKOEs. | ||
Morphological studies
Because the range of size normally expands over the capacity of any advanced instrument, the measurement of particle size must be taken at least with two corresponding techniques. Dynamic light scattering and transmission electron microscopy are the most recommended tools to measure the particle size, which is lower than 1 μm. Transmission electron microscopy was implemented to confirm the DLS data and to gain more information on the shape and size of the nanoemulsion oil globules. Fig. 6 shows the particle size of the nanoemulsion containing aripiprazole as determined by TEM. It was found that the average of particle size is smaller than 100 nm (between 65 nm and 80 nm) with almost spherical shape oil globules. It is observed that the particle shape is in accordance with the results measured using dynamic light scattering (DLS). | ||
| Fig. 6 TEM photomicrographs of the freshly prepared aripiprazole loaded nanoemulsion. The scale bar represents 200 nm. | ||
Conclusions
The compositions of the nanoemulsions containing aripiprazole, such as PKOEs, Tween 80, lecithin, glycerol, and water as effective variables, were modeled at different compositions using ANNs to define the desirable particle size of the nanoemulsions containing aripiprazole. To obtain the qualified network, five different algorithms, including QP, IBP, BBP, GA and LM were learned using training and testing data sets. The results of the learning program were 5 topologies: QP-5-4-1, BBP-5-10-1, IBP-5-11-1, GA-5-12-1 and LM-5-9-1. The performance of the topologies was optimized using RMSE, AAD and R2. The topology (QP-5-4-1) with the lowest RMSE was selected as the provisional network of the composition of the nanoemulsion containing aripiprazole. The importance of the variables included the Tween 80 content at 29.45% as the most important factor controlling the particle size, followed by water at 19.34%, glycerol at 18.57%, lecithin at 17.39%, and PKOE at 15.25%, which shows none of the variables was neglectable in this study. As a conclusion, the ANN is an efficient quantitative tool that is able to model the effective input variables used to predict the desirable particle size of a nanoemulsion containing aripiprazole.Conflict of interest
The authors have declared no conflict of interest.Acknowledgements
The author would like to express acknowledgment to University Putra Malaysia for granting this project under Research University Grant Scheme (RUGS).Notes and references
- M. Wilson, J. E. Helzer, H.-G. Hwu, E.-K. Yeh, M. McEvoy and J. E. Tipp, Am. J. Psychiatry, 1991, 148, 1697–1704 CrossRef PubMed.
- H. Li, J. Luo, C. Wang, S. Xie, X. Xu, X. Wang, W. Yu, N. Gu and J. M. Kane, Schizophr. Res. Treat., 2014, 157, 112–119 CrossRef PubMed.
- S. G. Potkin, A. R. Saha, M. J. Kujawa, W. H. Carson, M. Ali, E. Stock, J. Stringfellow, G. Ingenito and S. R. Marder, Arch. Gen. Psychiatry, 2003, 60, 681–690 CrossRef CAS PubMed.
- J. P. McEvoy, D. G. Daniel, W. H. Carson, R. D. McQuade and R. N. Marcus, J. Psychiatr. Res., 2007, 41, 895–905 CrossRef PubMed.
- H.-Y. Chan, W.-W. Lin, S.-K. Lin, T.-J. Hwang, T.-P. T. Su, S.-C. Chiang and H.-G. Hwu, J. Clin. Psychiatry, 2007, 68, 29–36 CrossRef CAS PubMed.
- N. J. Abbott, Cell. Mol. Neurobiol., 2005, 25, 5–23 CrossRef PubMed.
- N. J. Abbott, A. A. Patabendige, D. E. Dolman, S. R. Yusof and D. J. Begley, Neurobiol. Dis., 2010, 37, 13–25 CrossRef CAS PubMed.
- R. B. Mailman and V. Murthy, Curr. Pharm. Des., 2010, 16, 488 CrossRef CAS PubMed.
- M. Wood and C. Reavill, Expert Opin. Invest. Drugs, 2007, 16(6), 771–775 CrossRef CAS PubMed.
- E. Stip and V. Tourjman, Clin. Ther., 2010, 32, S3–S20 CrossRef CAS PubMed.
- J. Bhattacharjee and H. G. El-Sayeh, Aripiprazole versus typical antipsychotic drugs for schizophrenia, The Cochrane Library, 2008 Search PubMed.
- B. Chavez and R. A. Poveda, Ann. Pharmacother., 2006, 40, 2265–2268 CrossRef CAS PubMed.
- E. Merisko-Liversidge and G. G. Liversidge, Adv. Drug Delivery Rev., 2011, 63, 427–440 CrossRef CAS PubMed.
- F. Shakeel and W. Ramadan, Colloids Surf., B, 2010, 75, 356–362 CrossRef CAS PubMed.
- P. Keng, M. Basri, M. Zakaria, M. A. Rahman, A. Ariff, R. A. Rahman and A. Salleh, Ind. Crops Prod., 2009, 29, 37–44 CrossRef CAS.
- N. Salim, M. Basri, M. B. Rahman, D. K. Abdullah and H. Basri, Int. J. Nanomed., 2012, 7, 4739 CAS.
- H. Nakajima, S. Tomomasa and M. Okabe, In Proceedings of First Emulsion Conference, 1993, Paris, pp. 1–11 Search PubMed.
- H. Nakajima, Surfactant Sci. Ser., 1997, 66, 175–197 CAS.
- C. Solans, P. Izquierdo, J. Nolla, N. Azemar and M. Garcia-Celma, Curr. Opin. Colloid Interface Sci., 2005, 10, 102–110 CrossRef CAS.
- S. M. Jafari, Y. He and B. Bhandari, Food Res. Int., 2007, 40, 862–873 CrossRef CAS.
- P. Walstra, Chem. Eng. Sci., 1993, 48, 333–349 CrossRef CAS.
- S.-Y. Lee, S.-J. Chun, I.-A. Kang and J.-Y. Park, J. Ind. Eng. Chem., 2009, 15, 50–55 CrossRef CAS.
- N. Anton, J.-P. Benoit and P. Saulnier, J. Controlled Release, 2008, 128, 185–199 CrossRef CAS PubMed.
- M. Rezaee, M. Basri, R. N. Z. R. A. Rahman, A. B. Salleh, N. Chaibakhsh and H. R. F. Masoumi, Ind. Crops Prod., 2014, 52, 506–511 CrossRef CAS.
- A. Amani, P. York, H. Chrystyn and B. J. Clark, Pharm. Res., 2010, 27, 37–45 CrossRef CAS PubMed.
- E. Akbari, Z. Buntat, A. Enzevaee, S. J. Mirazimiabarghouei, M. Bahadoran, A. Shahidi and A. Nikoukar, RSC Adv., 2014, 4, 36896–36904 RSC.
- H. R. F. Masoumi, A. Kassim, M. Basri, D. K. Abdullah and M. J. Haron, Molecules, 2011, 16, 5538–5549 CrossRef CAS PubMed.
- Y. Abdollahi, N. A. Sairi, M. K. Aroua, H. R. F. Masoumi, H. Jahangirian and Y. Alias, J. Ind. Eng. Chem., 2014, 25, 168–175 CrossRef.
- Y. Abdollahi, A. Zakaria, M. Abbasiyannejad, H. R. F. Masoumi, M. G. Moghaddam, K. A. Matori, H. Jahangirian and A. Keshavarzi, Chem. Cent. J., 2013, 7, 96 CrossRef PubMed.
- Y. Abdollahi, S. B. M. Said, N. A. Sairi, M. F. B. M. Sabri, A. Zakaria, E. Abouzari-lotf and M. Dorraj, J. Ind. Eng. Chem., 2015, 29, 400–407 CrossRef CAS.
- Y. Sun, Y. Peng, Y. Chen and A. J. Shukla, Adv. Drug Delivery Rev., 2003, 55, 1201–1215 CrossRef CAS PubMed.
- H. Geers and W. Witt, in Direct calculation of the volume based particle size distribution from PCS or PCCS measurements, Proceedings of the Particulate Systems Analysis, Stratford-upon-Avon, UK, 2008 Search PubMed.
- W. Witt, H. Geers and L. Aberle, Particulate Systems Analysis, Harrogate, UK, 2003 Search PubMed.
- R. D. Snee, J. Qual. Tech., 1971, 3, 159–169 Search PubMed.
- M. G. Moghaddam, F. B. H. Ahmad, M. Basri and M. B. A. Rahman, Electron. J. Biotechnol., 2010, 13, 3–4 Search PubMed.
- M. Dorraj, Y. Abdollahi, S. B. Mohd Said, M. F. Bin Mohd Sabri, N. A. Sairi, W. P. Meng and E. Abouzari-lotf, RSC Adv., 2015, 5, 21384–21395 RSC.
- S. Askari, R. Halladj and M. J. Azarhoosh, RSC Adv., 2015, 5, 52788–52800 RSC.
- M. Ghaedi, A. Ghaedi, E. Negintaji, A. Ansari and F. Mohammadi, J. Ind. Eng. Chem., 2014, 20, 4332–4343 CrossRef CAS.
- A. Ghaffari, H. Abdollahi, M. Khoshayand, I. S. Bozchalooi, A. Dadgar and M. Rafiee-Tehrani, Int. J. Pharm., 2006, 327, 126–138 CrossRef CAS PubMed.
- P. Shabanzadeh, R. Yusof and K. Shameli, J. Ind. Eng. Chem., 2015, 24, 42–50 CrossRef CAS.
- D. G. Garson, AI Expert, 1991, 6, 47–51 Search PubMed.
- M. Khare and S. S. Nagendra, Artificial neural networks in vehicular pollution modelling, Springer, 2006 Search PubMed.
- D. Salari, N. Daneshvar, F. Aghazadeh and A. Khataee, J. Hazard. Mater., 2005, 125, 205–210 CrossRef CAS PubMed.
- P. Kaur, V. K. Sangal and J. P. Kushwaha, RSC Adv., 2015, 5, 34663–34671 RSC.
- M. Ghaedi, S. Hajati, M. Zare and S. Y. Shajaripour Jaberi, RSC Adv., 2015, 5, 38939–38947 RSC.
| This journal is © The Royal Society of Chemistry 2016 |