Open Access Article
Open Access ArticleConductivity of individual Geobacter pili†
Ramesh Y.
Adhikari
a,
Nikhil S.
Malvankar‡
ab,
Mark T.
Tuominen
a and
Derek R.
Lovley
*b
aDepartment of Physics, University of Massachusetts, Amherst, Massachusetts 01003, USA
bDepartment of Microbiology, University of Massachusetts, Amherst, Massachusetts 01003, USA. E-mail: dlovley@microbio.umass.edu
First published on 15th January 2016
Abstract
The electrically conductive pili of Geobacter species have been proposed to play an important role in long-range electron transfer to Fe(III) oxides and other cells and have potential as a sustainable source of electrically conductive materials. Surprisingly, there have been no previous reports on the actual conductivity of individual pili, probably the most important parameter for evaluating mechanistic models of electron transport and pili function. Therefore, the conductivity of individual pili of Geobacter sulfureducens was measured with a low-noise nano-electrode measurement platform along regions of the pili that appeared to be cytochrome-free. Pilus conductivity was highly dependent upon pH with conductivity estimates of 188 ± 34 mS cm−1, 51 ± 19 mS cm−1, and 37 ± 15 μS cm−1 at pH 2, 7, and 10.5, respectively. The conductivities of pili from strain Aro-5, which expresses pili in which an alanine was substituted for each of five aromatic amino acids, were significantly lower than the wild-type pili. These results, and the previous finding that stacking of aromatic amino acids increases at low pH, suggest that aromatic amino acids play a key role in pilus conductivity. The conductivity of the G. sulfurreducens pili is comparable to conducting organic polymer wires of similar diameter and several bacterial filaments of substantially different composition. These results provide important parameters that should be accommodated in future models of G. sulfurreducens pilus conductivity and suggest strategies for enhancing pilus conductivity with genetic manipulation.
Protein-based materials comprised of natural amino acids are attractive candidates for molecular electronics due to their diverse optical, electrical, mechanical, and chemical properties,1,2 as well as low cost and absence of toxicity.3 Previous studies suggested that the proteinaceous pili of Geobacter sulfurreducens are biologically unique electronic materials because they can conduct electrons over μm distances with metallic-like conductivity.4,5 However, this concept has been challenged repeatedly on the basis of theoretical modelling or inferences from biofilm behaviour.6–12 Remarkably, amongst this controversy the most basic data requirement, an estimate of the conductivity of individual pili, has been missing.
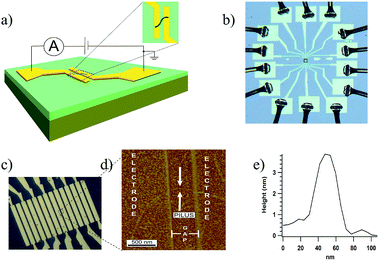
Therefore, a low-noise nano-electrode measurement platform was devised to directly measure the conductivity of individual pili (Fig. 1; see ESI† for additional details of construction). Arrays of gold electrodes, 2 μm wide and 10 μm long, separated by non-conducting gaps of 500 nm, were fabricated on n-doped silicon wafers with a 100 nm insulating layer of thermally grown oxide on the surface. The electrodes were connected to 100 μm × 100 μm pads for electrical contacts.
Pili from wild-type G. sulfurreducens, and strain Aro-5, a genetically altered strain in which key aromatic residues were replaced by alanine,13 were prepared as previously described.14 Buffer containing the pili was drop cast onto the electrode arrays. Excess buffer was removed, the samples rinsed with deionized water, and then gently air-dried (see ESI† for additional experimental details). This leaves pili in a hydrated state.15 Pili were located with atomic force microscopy. Occasionally, a single pilus bridging two electrodes was located (Fig. 1d). The c-type cytochrome OmcS, binds to the pili.16–19 Studies with mutant strains demonstrated that OmcS and similar c-type cytochromes on pili can be detected with AFM19 and the broad spacing between OmcS molecules often found on pili (100 s of nanometers)16,17 made it possible to conduct all conductivity measurements on pili in which no cytochromes were associated with the section of the pili bridging the gap between the electrodes. Height measurements (Fig. 1e) confirmed that each filament was a pilus, which have a diameter of 3 nm, without additional associated proteins and that the filaments were not flagella, which have a diameter of 12 nm.14
The contact pads corresponding to the two electrodes bridged by the individual pili were wire bonded with aluminium wire, connected to a printed circuit board, and the device was placed inside a double-shielded box with the inner box as a guard and the outer box as a ground (Fig. S1†). Additional details of experimental procedures are presented in the experimental section. All the measurements were performed in a temperature (22 °C) and humidity (55%) controlled clean room.
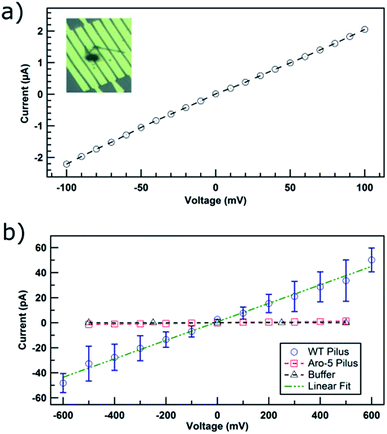
The device was initially evaluated with 150 nm diameter carbon nanotubes, as a positive control. The ohmic response (Fig. 2a) of current–voltage (IV) curve and the conductivity of 6 kS cm−1 were consistent with known properties of carbon nanotubes.20
At the physiologically relevant pH 7, individual wild-type pili of G. sulfurreducens, spanning the non-conducting gap between two electrodes, exhibited linear, ohmic behavior (Fig. 2b). Conductivity values (Table S1†) were calculated from the relation:
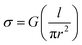 | (1) |
The current–voltage response of individual pili from strain Aro-5 was more similar to the response from buffer (Fig. 2b) without pili, yielding a conductivity estimate of 38 ± 1 μS cm−1, three orders of magnitude lower than wild-type pili. This result is consistent the conclusion, based on measurements on pili networks, that the pili from the Aro-5 strain poorly conduct electrons because they lack key aromatic amino acids required for electrical conductivity.13
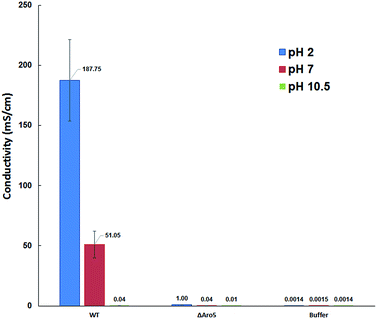
To further analyse the conductive properties of individual pili, conductance was measured at different pH. Increasing the pH to 10.5 dramatically lowered the conductivity of the wild-type pili to 37 ± 15 μS cm−1, whereas decreasing the pH to 2 substantially increased pilus conductivity to 188 ± 33 mS cm−1 (Fig. 3). In contrast, the conductivity of the buffer did not change significantly with pH. The change in pilus conductivity with pH is consistent with conformational changes that result in greater π–π stacking of aromatic amino acids at lower pH.5 This pH response of the individual pilus further suggested that the measured electronic conductivity is an intrinsic property of the pilus.
The pH also influenced the conductivity of the Aro-5 pili, but to a much lower extent than the wild-type (Fig. 3). This suggests that other factors such as charged amino acids may also contribute to conduction through pili, as previously suggested.5,8
Regardless of the mechanism of electron conduction, these measurements demonstrate that individual pili are electrically conductive. For comparison with other organic wires of similar diameter, the conductivity of the proton-doped pili is much higher than the conductivity (0.91 mS cm−1) of polypyrrole fibers of 80 nm diameter21 and compares favourably to the 90–600 mS cm−1 conductivity of PEDOT wires with a diameter of less than 10 nm.22 The G. sulfurreducens pili are substantially less conductive than the 1.34 kS cm−1 for carbon nanotubes with a diameter of 1.3 nm.23 Outer-membrane extensions of Shewanella oneidensis, which are a complex mixture of lipids, cytochromes, and potentially other proteins, form filaments when fixed with gluturaldehyde and critically point dried24 with conductivities of 60 mS cm−1 to 1 S cm−1.25 After a similar fixation procedure, the 50 nm diameter filaments of Rhodopseudmonas palustris, which are of unknown composition, have conductivities of 35–72 μS cm−1.26
Cross-linking proteins with gluturaldehyde has the potential to alter filament structure and thus conductivity. It will be of interest to assess the conductivity of the S. oneidensis and R. palustris filaments with the method described here. This method may also be useful for further analysis of conductivity along the length of other microbial filaments that are conductive across their diameter,27–29 often after chemical fixation.
Conclusions
The estimates of individual G. sulfurreducens pilus conductivity reported here provide a key piece of data that is needed for assessing the diverse proposed models for the conductivity of G. sulfurreducens pili.6–10,12 Models that include a role of c-type cytochromes in electron conduction along the length of the pili,7,9,10 must account for conductivity measured here in reaches of the pili that were cytochrome free. The major impact of proton-doping on conductivity should also be accommodated.The conductivity of G. sulfurreducens pili suggests that they may be useful electronic materials with the advantages that they can readily be mass-produced in a sustainable manner and they do not contain toxic components. The pili function in water, are highly chemically stable, and their properties can be readily be genetically modified. The strong dependence of pilus conductivity on pH and their high aspect ratio suggests that G. sulfurreducens pili might have applications as highly sensitive pH or other environmental sensors. The finding that there are substantial increases in pilus conductivity associated with proton doping, which increases stacking of aromatic amino acids,5 suggests that genetic manipulation to further increase interactions of aromatic amino acids may enhance pilus conductivity.
Acknowledgements
We thank John Nicholson for help with nanofabrication and the staff of Keithley Instruments for advice on low-level measurements. We also thank Yiming Chen and Su-Wei Chang for help with wire bonding and Martin Muthee for providing carbon nanotubes. This research was supported by the Office of Naval Research (grant no. N00014-12-1-0229 and N00014-13-1-0550) and National Science Foundation (NSF) Nanoscale Science and Engineering Center (NSEC) Center for Hierarchical Manufacturing (CHM) (grant no. CMMI-1025020). Nikhil S. Malvankar holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.Notes and references
- N. Amdursky, D. Marchak, L. Sepunaru, I. Pecht, M. Sheves and D. Cahen, Adv. Mater., 2014, 26, 7142–7161 CrossRef CAS PubMed.
- J. Gosline, M. Lillie, E. Carrington, P. Guerette, C. Ortlepp and K. Savage, Philos. Trans. R. Soc., B, 2002, 357, 121–132 CrossRef CAS PubMed.
- C. A. E. Hauser and S. Zhang, Nature, 2010, 468, 516–517 CrossRef CAS PubMed.
- D. R. Lovley and N. S. Malvankar, Environ. Microbiol., 2015, 17, 2209–2215 CrossRef CAS PubMed.
- N. S. Malvankar, M. Vargas, K. Nevin, P.-L. Tremblay, K. Evans-Lutterodt, D. Nykypanchuk, E. Martz, M. T. Tuominen and D. R. Lovley, mBio, 2015, 6, e00084 CrossRef PubMed.
- P. N. Reardon and K. T. Mueller, J. Biol. Chem., 2013, 288, 29260–29266 CrossRef CAS PubMed.
- P. S. Bonanni, D. Massazza and J. P. Busalmen, Phys. Chem. Chem. Phys., 2013, 15, 10300–10306 RSC.
- G. T. Feliciano, A. J. R. Da Silva, G. Reguera and E. Artacho, J. Phys. Chem. A, 2012, 116, 8023–8030 CrossRef CAS PubMed.
- H. Yan, C. Chuang, A. Zhugayevych, S. Tretiak, F. W. Dahlquist and G. C. Bazan, Adv. Mater., 2015, 27, 1908–1911 CrossRef CAS PubMed.
- S. M. Strycharz-Glaven, R. M. Snider, A. Guiseppi-Elie and L. M. Tender, Energy Environ. Sci., 2011, 4, 4366 CAS.
- M. D. Yates, J. Golden, J. Roy, S. M. Strycharz-Glaven, S. Tsoi, J. Erickson, M. Y. El-Naggar, S. Calabrese Barton and L. Tender, Phys. Chem. Chem. Phys., 2015, 17, 32564–32570 RSC.
- N. Lebedev, S. Mahmud, I. Griva, A. Blom and L. M. Tender, J. Polym. Sci., Part B: Polym. Phys., 2015, 53, 1706–1717 CrossRef CAS.
- M. Vargas, N. S. Malvankar, P.-L. Tremblay, C. Leang, J. A. Smith, P. Patel, O. Synoeyenbos-West, K. P. Nevin and D. R. Lovley, mBio, 2013, 4, e00105–13 CrossRef PubMed.
- N. S. Malvankar, M. Vargas, K. P. Nevin, A. E. Franks, C. Leang, B. C. Kim, K. Inoue, T. Mester, S. F. Covalla, J. P. Johnson, V. M. Rotello, M. T. Tuominen and D. R. Lovley, Nat. Nanotechnol., 2011, 6, 573–579 CrossRef PubMed.
- N. H. Thomson, J. Microsc., 2005, 217, 193–199 CrossRef CAS PubMed.
- C. Leang, X. Qian, T. Mester and D. R. Lovley, Appl. Environ. Microbiol., 2010, 76, 4080–4084 CrossRef CAS PubMed.
- N. S. Malvankar, M. T. Tuominen and D. R. Lovley, Energy Environ. Sci., 2012, 5, 8651 CAS.
- N. S. Malvankar, S. E. Yalcin, M. T. Tuominen and D. R. Lovley, Nat. Nanotechnol., 2014, 9, 1012–1017 CrossRef CAS PubMed.
- J. Yun, N. S. Malvankar, T. Ueki and D. R. Lovley, ISME J., 2015, 1–11 Search PubMed.
- T. W. Ebbesen, H. J. Lezec, H. Hiura, J. W. Bennett, H. F. Ghaemi and T. Thio, Nature, 1996, 382, 54–56 CrossRef CAS.
- L. Liu, Y. Zhao, N. Jia, Q. Zhou, C. Zhao, M. Yan and Z. Jiang, Thin Solid Films, 2006, 503, 241–245 CrossRef CAS.
- S. Samitsu, T. Shimomura, K. Ito, M. Fujimori, S. Heike and T. Hashizume, Appl. Phys. Lett., 2005, 86, 1–3 CrossRef.
- S. Tans, M. Devoret, H. Dai, A. Thess, R. E. Smalley, L. J. Georliga and C. Dekker, Nature, 1997, 386, 474–477 CrossRef CAS.
- S. Pirbadian, S. E. Barchinger, K. M. Leung, H. S. Byun, Y. Jangir, R. A. Bouhenni, S. B. Reed, M. F. Romine, D. A. Saffarini, L. Shi, Y. A. Gorby, J. H. Golbeck and M. Y. El-Naggar, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12883–12888 CrossRef CAS PubMed.
- M. Y. El-Naggar, G. Wanger, K. M. Leung, T. D. Yuzvinsky, G. Southam, J. Yang, W. M. Lau, K. H. Nealson and Y. A. Gorby, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18127–18131 CrossRef CAS PubMed.
- K. Venkidusamy, M. Megharaj, U. Schröder, F. Karouta, S. V. Mohan and R. Naidu, RSC Adv., 2015, 5, 100790–100798 RSC.
- Y. Li and H. Li, J. Basic Microbiol., 2014, 54, 226–231 CrossRef CAS PubMed.
- L. Castro, M. Vera, J. Á. Muñoz, M. L. Blázquez, F. González, W. Sand and A. Ballester, Res. Microbiol., 2014, 165, 794–802 CrossRef CAS PubMed.
- S. Sure, A. A. J. Torriero, A. Gaur, L. H. Li, Y. Chen, C. Tripathi, A. Adholeya, M. L. Ackland and M. Kochar, Antonie van Leeuwenhoek, 2015, 108, 1213–1225 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental methods. Fig. S1: double shielding system for low current measurements. Fig. S2: steady state current measurement. Table S1 experimental conductance and conductivity values. See DOI: 10.1039/c5ra28092c |
| ‡ Present address: Department of Molecular Biophysics and Biochemistry, Microbial Sciences Institute, Yale University, Connecticut, 06516, USA. |
| This journal is © The Royal Society of Chemistry 2016 |