Correction function for biased results due to matrix effects in residue analysis of beta-agonists in porcine tissues and urine with LC-MS/MS†
Abstract
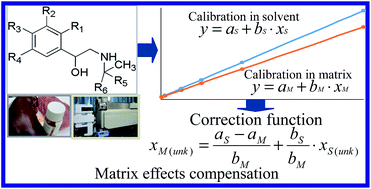
In the residue analysis of nine β-agonists, namely salbutamol, terbutaline, cimaterol, fenoterol, clorprenaline, ractopamine, tulobuterol, clenbuterol and penbuterol in porcine liver, muscle and urine with liquid chromatography tandem mass spectrometry (LC-MS/MS), solvent calibration (SC) and matrix-matched calibration (MC) were compared using analysis of covariance to estimate the matrix effects (MEs) for all the analytes. Significant differences (P < 0.05) between the SC and MC were found for most analytes, indicating that the matrix induces systematic or proportional errors in the quantification of those analytes. In such cases, a correction function (CF) is proposed, with which the real concentration of analytes could be predicted using a SC. The results of the validation study showed that recoveries of the analytes calculated using the CF were comparable to those calculated using the MC, suggesting that the CF could be satisfactorily applied to the correction of MEs and the quantification of β-agonists in their residue analysis in porcine liver, muscle and urine by LC-MS/MS.

 Please wait while we load your content...
Please wait while we load your content...