Highly functionalized heterogeneous dendrigraft catalysts with peripheral copper moieties for the facile synthesis of 2-substituted benzimidazoles and 2,2-disubstituted benzimidazoles†
G. Smitha and
K. Sreekumar*
Department of Applied Chemistry, Cochin University of Science and Technoogy, Kochi-22, Kerala, India. E-mail: kskpolymer.cusat@gmail.com; Tel: +91-484-2575804 Tel: +91-9447121530 Tel: +91-9447053021
First published on 26th January 2016
Abstract
The synthesis of dendrigraft amidoamine polymers with glycerol initiated polyepichlorohydrin cores has been demonstrated on a Merrifield resin support to achieve heterogenosity for catalytic applications. In earlier studies of supported dendritic systems, no effort has been initiated to synthesize a dendritic polymer with high functionality at a low generation. The copper complexes of the Gn (n = 0, 1, 2) dendrigraft polymers were found to be excellent catalysts for the synthesis of benzimidazole derivatives via reactions between o-phenylenediamine and aldehydes. Aliphatic and cyclic ketones also showed excellent conjugation with o-phenylenediamine. A detailed study of the synthesis of 2-substituted benzimidazoles and 2,2-disubstituted benzimidazoles was performed with dendrigraft G2 copper catalyst. The reusability of the catalyst was examined for five consecutive steps, and no significant loss of catalytic activity was observed. The catalyst possessed the following green attributes: the final oxidation step was conducted using air, only a small amount of catalyst was needed to drive the reaction, and ethanol was used as the solvent under room temperature conditions.
1. Introduction
Recently, great attention has been directed towards the synthesis of dendrigraft or dendronized polymers.1 A high amount of functionality can be achieved with these polymers in comparison with dendrimers. In the case of dendrigraft polymers,2 polymers are grafted on another polymer, and in dendronized polymers, the focal points of the dendrons are connected to pending functional groups. This occurs at every repeating unit along the polymer backbone. The result is a special case of a graft copolymer called a comb polymer.3 This polymer has the peculiar feature that all the side chains are dendrons. Polymers with this architecture are referred to as dendronized polymers, or ‘denpols’.4 These terms are generally assigned to polymers having a linear type core. In the present context, it is believed that the term dendrigraft polymer is more appropriate here. This is because the dendrons are grafted on the polymeric chain, whether linear or branched. This differs from the situation of grafting polymeric chains, as in the case of Tomalia's3 or Gauthier's5 dendrigraft polymers, and it would be better to call dendronized polymers dendrigrafts, or group them under the dendrigraft family. Most of the reported dendronized polymers were synthesized from linear polymeric cores.1a,6 There are few reports of dendronized polymers synthesized from cyclic or branched polymeric cores.7 However, the synthesis of these polymers is practically difficult, due to the lengthy purification procedure after every stage. Therefore, we have attempted to synthesize dendrigraft or dendronized polymers with branched polymeric cores on a solid resin support using Merrifield's solid phase synthesis concept.8 The thought process behind the synthesis of this supported, highly functionalized polymer was that a high amount of functionality would be helpful to achieve sustainable catalysis.During the last decade, researchers working with dendrimers have switched their focus from the synthetic attributes of said dendrimers to their applications. Thus, metallodendrimers are gaining attention from a materials science perspective because of their distinctive physical properties and significant structural diversity, which lead to promising photophysical and catalytic applications.9 Several catalytic investigations based on metallodendrimers, including those based on polyamidoamine10 and polypropyleneimine,11 have been reported.12 Scandium and palladium modified dendritic complexes for the Friedel–Crafts reaction12f and Suzuki coupling,12g respectively, have been reported recently. The efficiency of these catalysts is overshadowed by the difficulty of their synthesis and purification. Polymer supported synthesis helps to overcome this problem, and a number of polymer supported dendritic catalysts have been reported.8a,g,10d,13 However, surprisingly, a polymer supported dendrigraft or dendronized polymer has not yet been reported. Herein, we report copper complexes of dendrigraft polymers with glycerol initiated polyepichlorohydrin cores on an organic polymeric support, Merrifield resin, for the synthesis of benzimidazole derivatives via the conjugation of o-phenylenediamine with aldehydes or ketones.
Benzimidazole derivatives have been found to possess enormous therapeutic applications, including antiviral, antihypertensive, antiulcer, antifungal, antihistaminic, and anticancer activities.14 For example, the glycinamide-containing benzimidazoles have valuable pharmacological properties such as anti-CCR2 and antithrombotic activities.15 Several 3-benzimidazol-2-yl-1H-quinoxalin-2-ones coordinate with Ru or Os to obtain complexes with antiproliferative activities.16 The discovery of this class of drugs provides an outstanding case history of modern drug development and also points out the unpredictability of pharmacological activity from the structural modification of a prototype drug molecule.17 Imidazole moieties also serve as important intermediates in numerous organic reactions18 and have been used as important ligands for transition metals in various organic transformations.19 Benzimidazole derivatives have been also used in material chemistry.20 Due to their wide applications, the preparation of benzimidazole derivatives has gained considerable attention in recent years.
However, the synthesis of benzimidazole derivatives via the conjugation of an o-aromatic diamine with an aldehyde or a ketone by means of a single catalyst is uncommon. Specifically, reported procedures of benzimidazole synthesis involve the combination of an o-aromatic diamine with either an aldehyde or a ketone. Herein, we have used our highly functionalized single catalyst for the synthesis of both 2-substituted and 2,2-disubstituted benzimidazoles.
2. Experimental
2.1 Materials and methods
Chloromethyl polystyrene (1% DVB crosslinked, 100–200 mesh) was donated from Thermax India Ltd. as a gift sample. This was washed with methanol, dioxane and acetone and dried under vacuum. Sodium hydride, tetrabutylammonium bromide, p-toluenesulfonyl chloride, sodium azide, LiAlH4, methyl acrylate, ethylene diamine, anhydrous copper acetate, o-phenylenediamine, aldehydes and ketones were all purchased from local vendors and were used as received. Absolute ethanol was obtained from Sd. Fine India Ltd. and was used as received. All other solvents were distilled by standard procedures prior to use.The IR spectra were obtained with samples as KBr pellets using a JASCO 4100 FTIR spectrometer. The spectra were obtained at ambient temperature by making pressed pellets of the compounds. Solid state 13C NMR spectra were obtained with a Bruker 400 MHz instrument with a resonance frequency of 75.5 MHz (NCL, Pune). The 1H and 13C NMR spectra of the compounds were obtained on a 300 or 400 MHz Bruker advanced DPX spectrometer using CDCl3 or DMSO-d6 as the solvent and TMS as an internal standard (NIIST, Trivandrum, STIC, CUSAT). Melting points were determined in an open capillary tube on a Buchi Melting Point B-540 apparatus and are uncorrected. GC analysis was carried out on a Varian 1200 L Single Quadrupole gas chromatograph. The SEM characterization was carried out using a JEOL Model Scanning Electron Micrograph with an attached energy-dispersive X-ray detector. Scanning was performed in the 1–20 μm range, and the images were taken at a magnification of 15–20 kV. The data were obtained using INCA software. Standardization of the data analysis is an integral part of the SEM-EDX instrument employed (STIC, CUSAT). Thermogravimetric analysis was performed using a Perkin Elmer Diamond TG/DTA system at a heating rate of 10 °C min−1 under an atmosphere of nitrogen using an aluminium pan. The magnetic susceptibilities of the complexes were measured by the Gouy method, using Hg[Co(NCS)] as the calibrant (IIT, Chennai). The copper content was estimated by atomic emission spectroscopy (ICP-AES Thermo Electron IRIS INTREPID IIXSP DUO). The samples were prepared by first igniting them in a Bunsen flame. Subsequently, the residue was acid digested followed by evaporation to dryness. 20 mL of distilled water was added to the dry mass, and the metal content was estimated by AES. The metal contents were also obtained from EDX analysis. The diffuse reflectance UV-Vis spectra of the solid samples were recorded using a UV-Vis-NIR Ocean Optics SD 2000 spectrophotometer equipped with a diffuse reflectance accessory. The powder X-ray diffraction (XRD) patterns were obtained on a Bruker AXS D8 Advance X-ray diffractometer (STIC, CUSAT). XPS measurements were carried out using a multi-probe system (Omicron Nanotechnology, Germany) equipped with a dual Mg/Al X-ray source and a hemispherical analyzer operating in constant analyzer energy (CAE) mode. The Mg Kα X-ray source was operated at 300 W and 15 kV. The base pressure in the analyzing chamber was maintained at 1 × 10−10 mbar. Charging of the samples was corrected by setting the binding energy of the adventitious carbon (C 1s) at 284.6 eV (AIMS, Cochin). EPR analysis was performed using a JES-FA200 ESR spectrometer (IIT, Mumbai). The Merrifield resin supported polymers were synthesized by a reported procedure with minor modifications.
2.2 Preparation of polyepichlorohydrin – PECH
Epichlorohydrin (11.742 mL, 0.15 mol) was added through a dropping funnel to a cooled reaction mixture containing dichloromethane (10 mL), glycerol (0.73 mL, 0.01 mol) and BF3-etherate (1.256 mL, 0.01 mol) with constant stirring. The reaction mixture was stirred for 24 h at 30 °C. After completion of the reaction, the reaction mixture was transferred to a separating funnel and washed with saturated sodium carbonate solution followed by distilled water. The solvent was removed under vacuum.Yield: 15 g; colourless viscous liquid; mp (GPC): 1589; polydispersity: 1.06; IR (cm−1); 3490, 2850, 1380, 1100, 750; 1H NMR (400 MHz, CDCl3): 3.2–4.3 (m, CH2, CH & OH), 2.1 (m, CH, CH2), 1.78 (m, CH, CH2), 1.68 (s, OH), 1.15–1.17 (m, CH, CH2) ppm.
2.3 Coupling of PECH to the resin
Sodium hydride (1 g, 0.04 mol) was added to a stirred solution of PECH (5.0 g) in dry DMF (10 mL) at 0 °C. After 2 h, Merrifield resin (1.0 g) and tetrabutylammonium bromide (216 mg, 0.62 mmol) were added, and the mixture was shaken at room temperature (30 °C) for 20 h. The reaction was quenched by the addition of water (20 mL) and the resin was filtered, followed by washing with water and drying to constant weight under vacuum to yield the PECH loaded resin. The unreacted PECH was removed by Soxhlet extraction with dichloromethane and dried under vacuum for 5 h.Yield: 4.3 g; chlorine capacity: 12.863 mmol g−1; hydroxyl group capacity: 1.43 mmol g−1. IR (cm−1): 3430 (νstretch(–OH)), 3056, 2925, 1602, 1535, 1448, 1360, 1107 (νstretch(C–O–C)), 695; 13C NMR (75.5 MHz): 19, 38.6, 42.2, 69.9 (–CH2–O–), 130.2, 148.6 ppm.
2.4 Tosylation of hydroxyl groups
The Merrifield resin supported PECH (1.0 g) was dispersed in pyridine and cooled to −5 °C. A cold solution of p-toluenesulfonyl chloride (0.7 g, 0.004 mol) in 5 mL pyridine was added slowly. Upon completion of the addition, the temperature was maintained at −5 °C for 30 minutes and allowed to come to room temperature (30 °C) overnight. The contents of the flask were quenched with ice water. The resin was filtered and washed with ice water and ethanol. The resin obtained was dried under vacuum for 5 h.Yield: 1.06 g; %S: 1.63; IR (cm−1); 3054, 2935, 1678, 1528, 1421, 1457, 1260 (νstretch(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1182 (νstretch(S
O)), 1182 (νstretch(S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)), 1105, 1034, 700; 13C NMR (75.5 MHz): 19, 21 (CH3), 38.6, 42.2, 69.9, 128–130 (C6H4), 146 (C6H4) ppm.
O)), 1105, 1034, 700; 13C NMR (75.5 MHz): 19, 21 (CH3), 38.6, 42.2, 69.9, 128–130 (C6H4), 146 (C6H4) ppm.
2.5 Synthesis of polyazide
The Merrifield resin supported tosylated PECH (1.0 g) was allowed to swell in 5 mL DMF; sodium azide (0.455 g, 0.007 mol) in 5 mL DMF was added and the mixture was heated at 85 to 90 °C for 24 h. After the reaction, the resin was filtered and was washed with water. The resin obtained was dried under vacuum for 5 h.Yield: 1.34 g; IR (cm−1); 3050, 2850, 2100 (νstretch(N3)), 1600, 1474, 1120, 1025; 13C NMR (75.5 MHz): 19, 20.8, 42.3, 49 (CH2–N3), 70.0, 125.8, 133.6, 148.2 ppm.
2.6 Synthesis of polyamine (synthesis of G0 polymer)
The Merrifield resin supported polyazide (1 g) was suspended in dry THF taken in a RB flask and maintained at 0 °C in an ice bath. Slurry of LiAlH4 (0.304 g, 0.008 mol) in dry THF was added dropwise to the reaction mixture with stirring. The reaction mixture was maintained at 0 °C for 1 h. The temperature was slowly brought to 50 °C. The mixture was stirred at 50 °C for two days to ensure complete reduction. Excess LiAlH4 was removed by adding ethyl acetate. The solution was filtered and washed with water. The resin was dried under vacuum for 5 h.Yield: 0.83 g; amine capacity: 13.45 mmol g−1. IR (cm−1); 3477 (νstretch(NH)), 1568 (νbend(NH)), 1340, 1047; 13C NMR (75.5 MHz): 18.2, 20.8, 42 (CH2NH2), 71, 125.8, 133, 147.3 ppm.
2.7 Michael addition reaction (synthesis of G0.5 polymer)
The Merrifield resin supported G0 polyamine (0.5 g) was added in portions to a RB flask containing methyl acrylate (0.68 mol, 8 mL) and methanol (5 mL) at room temperature (30 °C) with stirring. The reaction mixture was stirred at room temperature for 7 days under an atmosphere of nitrogen. The reaction was monitored using the Kaiser ninhydrin test. After the reaction, excess methyl acrylate was decanted; the resin was filtered and washed with water. The resin was dried under vacuum for 5 h.Yield: 3.2 g; IR (cm−1): 3426, 2912, 1741 (νstretch(COO)), 1310, 1265, 1056; 13C NMR (75.5 MHz); 20, 45, 65, 125, 144, 169.8 (COO) ppm.
2.8 Procedure for transamination (synthesis of G1 polymer)
The Merrifield resin supported G0.5 polymer (1.0 g) was added in small portions with stirring to a mixture of ethylene diamine (0.135 mol, 10 mL) and methanol (10 mL) in a RB flask, which was then cooled to 0 °C in an ice salt bath. The reaction mixture was stirred at 0 °C for 1 h and the temperature was allowed to increase to room temperature (30 °C); the mixture was stirred at room temperature for 7 days to ensure complete reaction. After the completion of the reaction, the resin was filtered and washed with water. The resin was dried under vacuum for 5 h.Yield: 1.7 g; amine capacity: 22.13 mmol g−1; IR (cm−1): 3477, 2935, 1655 (νstretch(CONH)), 1375, 1056; 13C NMR (75.5 MHz); 20, 45, 65, 125, 144, 167.4 (CONH) ppm.
2.9 Michael addition reaction (synthesis of G1.5 polymer)
The Merrifield resin supported G1 polymer (1.0 g) was added in portions to a RB flask containing methyl acrylate (0.27 mol, 25 mL) and methanol (30 mL) at room temperature (30 °C) with stirring. The reaction mixture was stirred at 30 °C for 10 days under an atmosphere of nitrogen to ensure complete reaction. The reaction was monitored using the Kaiser ninhydrin test. After the reaction, the excess methyl acrylate was decanted; the solution was filtered and the resin was washed with water. The resin was dried under vacuum for 5 h.Yield: 3.34 g; IR (cm−1): 3475, 2984, 1747 (νstretch(COO)), 1376, 1056; 13C NMR (75.5 MHz); 20, 45, 65, 125, 144, 167.3 (CONH), 169.7 (COO) ppm.
2.10 Procedure for transamination (synthesis of G2 polymer)
The Merrifield resin supported G1.5 polymer (1.0 g) was added in small portions with stirring to a mixture of ethylene diamine (0.27 mol, 20 mL) and methanol (20 mL) in a RB flask, which was then cooled to 0 °C in an ice salt bath. The reaction mixture was stirred at 0 °C for 1 h and the temperature was allowed to increase to room temperature (30 °C), followed by stirring at room temperature for 7 days to ensure complete reaction. After the completion of the reaction, the resin beads were filtered and washed with water. The resin was dried under vacuum for 5 h.Yield: 1.8 g; amine capacity: 30.24 mmol g−1; IR (cm−1): 3473, 2958, 1645 (νstretch(CONH)), 1375, 1041; 13C NMR (75.5 MHz); 20, 48, 65, 125, 144, 167.1 (CONH), 167.4 (CONH) ppm.
2.11 Synthesis of copper complex of dendrigraft GLR-G2 polymer with a glycerol initiated polyepichlorohydrin core
A 50 mL round bottom flask was charged with 250 mg of Merrifield resin supported GLR-Gn (n = 0, 1 and 2) polymer having “x” mmols of amine capacity. The resin was allowed to swell in 5 mL DMF for 2 h. A quantitative amount corresponding to “x” mmols of anhydrous copper acetate in 10 mL methanol was added to the reaction flask. The reaction mixture was stirred at 50 °C for two days (48 h). The polymer was filtered and washed with water. The filtrate and washings were collected and concentrated. This concentrated solution was used for the estimation of metal ions by standard methods. The polymer-supported metal complex was dried under vacuum for 5 h.2.12 General procedure for the synthesis of benzimidazole derivatives from aldehydes/ketones
A 25 mL RB flask with a side inlet was charged with o-phenylenediamine (1.0 mmol), aldehyde/ketone (1.0/1.2 mmol), ethanol (3.0 mL) and catalyst GLR-G2-Cu (0.007 mol%, 10 mg). The mixture was stirred at room temperature (30 °C) under air. After the reaction was completed (TLC and GCMS determination), the resulting solution was filtered, washed with ethyl acetate, and concentrated by rotary evaporator, and the residue was purified by column chromatography on silica gel using hexane and ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to provide the desired target product.
1) as the eluent to provide the desired target product.
2.13 Test for catalyst heterogeneity
To confirm whether the conjugation reactions occurred via a heterogeneous catalytic process, and to identify any leaching of the metal complex from the polymer-bound dendrigraft catalyst into the reaction medium during the synthesis of benzimidazole derivatives, reactions were conducted according to the abovementioned procedure. After completion of the reaction, the catalyst was filtered and the filtrate obtained was extracted with ethyl acetate and water. The aqueous layer was subsequently treated with another set of reactants and the reactions were allowed to continue for a specified time. There was no formation of the product. This suggests that the reaction does not proceed after removal of the catalyst. Moreover, the presence of copper could not be detected when the filtrate obtained after separating the solid catalysts by filtration was subjected to ICP-AES analysis. These facts ruled out the possibility of the copper species leaching out of the catalyst, which proves the heterogeneous nature of the catalytic process.2.14 Regeneration and recycling of the catalyst
The reusability of the catalyst for subsequent catalytic cycles was examined using cyclohexanone/benzaldehyde and o-phenylenediamine as the substrate. In the oxidative cyclisation reaction, after the completion of the reaction, the solid catalyst was separated from the reaction mixture by filtration, washed with ethyl acetate and dried under vacuum for about 5 h. The dried solid catalyst was weighed and added to a fresh reaction mixture of cyclohexanone (1.2 mmol)/benzaldehyde (1.0 mmol), o-phenylenediamine (1.0 mmol) and ethanol (3 mL). The progress of the reaction was monitored by thin layer chromatography (TLC) and GCMS. The procedure for the abovementioned system was repeated for five reaction cycles.3. Results and discussion
The Merrifield resin supported dendrigraft amidoamine polymer was synthesized using the schematic procedure shown in Scheme 1. The branched hydroxyl terminated polyepichlorohydrin (PECH) was prepared by the ring opening polymerization of the oxirane group in epichlorohydrin (ECH) in the presence of glycerol by an activated monomer mechanism (AMM).21 The polymer was a colorless viscous liquid. From GPC, the molecular weight of PECH was found to be 1589 with a polydispersity of 1.06. The PECH was coupled to the resin via sodium hydride and tetrabutylammonium bromide.22 After coupling, quantitative estimation shows the amount of chlorine as 12.863 mmol g−1. The EDX spectrum shows carbon, oxygen and chlorine as the main constituents of the polymer (ESI†). The 13C NMR spectrum of PECH loaded Merrifield resin shows a broad peak around 69.9 ppm (Fig. 1). Moreover, the peak indicating the hydroxyl group after the loading of PECH on Merrifield resin can be observed at 3430 cm−1 in the IR spectrum (ESI†).The hydroxyl groups of the PECH loaded resin were estimated quantitatively and were found to be 1.43 mmol g−1 of the polymer. However, it was necessary to protect the hydroxyl groups of the polymer to avoid unwanted reactions and to promote dendron growth. This problem can be managed by producing glycidyl azide polymer without terminal hydroxyl groups. This polymer is produced by reacting supported polyepichlorohydrin with p-toluenesulfonyl chloride (TsCl) in pyridine, and reacting the resulting tosylated polyepichlorohydrin with sodium azide in dimethylformamide.23 After tosylation, the percentage of sulphur was found to be 1.63% from CHNS data. Thus, azidation of tosylated PECH with sodium azide gives the corresponding azide polymer in quantitative yield.23,24 An intense band at 2100 cm−1 in the IR spectrum confirms the presence of the azide functionality (ESI†). The colour of the resin changed from off-white to brown. The polymeric diazide, upon reduction with lithium aluminium hydride (LiAlH4) in dry THF, was converted to the polyamine (GLR-G0).11f,22a The band at 2100 cm−1 in the IR spectrum disappeared and a band at 3477 cm−1 showed the presence of the amine functionality. The positive Kaiser ninhydrin test confirmed the reduction of polyazide to polyamine. The quantitative estimation showed the presence of 13.45 mmol of amine per gram of the resin. In the 13C NMR spectra of tosylated PECH, polyazide and G0 polyamine, no characteristic peak can be observed; this is because the peaks for the tosyl carbons, –CH2N3 and –CH2NH2 merge within the region of the Merrifield resin. In the TG-DTG curve of GLR-G0 polymer, the first mass loss of about 25% around 100–300 °C was due to the elimination of amines as molecular nitrogen. The second mass loss of about 36% at 382 °C was due to the degradation of resin loaded polyether25 (Fig. 2). This information was obtained in comparison with the TG of PECH loaded resin (ESI†).
Michael addition of the GLR-G0 polyamine, which contains terminal amine groups, with methyl acrylate resulted in GLR-G0.5 polymer with terminal ester groups.26 As a result of this reaction, a peak at 1741 cm−1 due to the ester carbonyl can be observed in the IR spectrum (ESI†). The signal corresponding to 169.8 ppm in the 13C NMR spectrum of GLR-G0.5 is due to the carbonyl carbon of the ester functionality (Fig. 3). The abovementioned polymer was coupled with ethylene diamine to achieve a new amino terminated resin.26 The amidoamine G1 polymer (GLR-G1) shows bands at 1655 cm−1 and 3482 cm−1 in the IR spectrum, attributable to the carbonyl and amine moieties of the polymer, respectively (ESI†). The quantitative estimation showed the presence of 22.13 mmol of amine per gram of the resin. In the 13C NMR spectrum, the peak corresponding to 167.4 ppm corresponds to carbonyl carbon of the amide functionality (ESI†).
In the TG curve of the GLR-G1 resin, two mass loss steps were observed. The first decomposition corresponded to nearly 30% mass loss at a temperature around 212.97 °C. The second stage mass loss of 40% was observed around 410 °C (Fig. 4). The synthesis procedure was repeated to obtain the GLR-G2 dendrigraft polymer.26 Upon subsequent Michael addition of the GLR-G1 polyamine to methyl acrylate, a peak appeared at 1747 cm−1 in the IR spectrum (ESI†). In the 13C NMR spectrum of GLR-G1.5, the signals corresponding to 169.7 ppm and 167.3 ppm are due to the carbonyl carbons of the ester and amide functionalities, respectively (ESI†). Similarly, subsequent amide coupling with ethylene diamine gives the amidoamine G2 polymer (GLR-G2). The peaks at 1645 cm−1 and 3482 cm−1 in the IR spectrum of the G2 polymer are due to the carbonyl and amine moieties of the polymer, respectively (ESI†).
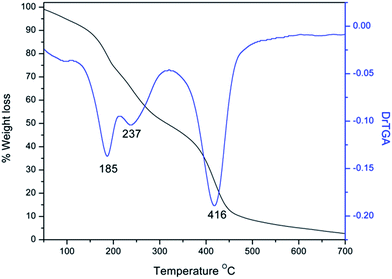
The signals at 167.1 ppm and 167.4 ppm in the 13C NMR spectrum of the GLR-G2 polymer were attributed to the carbonyl carbons of the different amide functionalities (ESI†). The quantitative estimation of amine functionality in the GLR-G2 polymer was found to be 30.24 mmol g−1, greater than the amine content of the GLR-G1 polymer, which was again greater than that of the GLR-G0 polymer; this signifies the amount of growth on the polyether backbone. The TG curve of the GLR-G2 polymer shows a similar decomposition pathway to that of GLR-G1 polymer; however, a mass loss of 42% was observed between 120 and 320 °C and a 45% mass loss was observed at 416 °C (Fig. 5). Thus, the percentage weight loss increased in the TG profile from G0 to G2, which supports the growth of the dendrons with each generation.
Copper complexes are well known for their catalytic activity. Recently, copper and other metal complexes of dendritic and nondendritic polymers as catalysts for organic reactions have been developed in this laboratory.10d,13a,27 In the present study, we have attempted to develop copper complexes of dendrigraft polymers with glycerol initiated polyepichlorohydrin cores such as GLR-G2-Cu (Fig. 6) to effectively catalyze different organic reactions in an environmentally benign manner. Chloromethylated polystyrene crosslinked with 1% DVB was used as the support mainly owing to its superior flexibility, which is known to facilitate the grafting of metal ions via dendritic ligands.28 The copper complex of each GLR-Gn (n = 0, 1 and 2) polymer was synthesized by adopting the schematic procedure shown in Scheme 2. For the complexation of dendrigraft polymer with copper, the copper salt and solvent were optimized. It was found that copper acetate in methanol at a temperature of 50 °C was the best condition for the complexation of copper with the GLR-Gn (n = 0, 1 and 2) dendritic polymers. Factors, such as the maintenance of the required contact time and temperature, were also found to be important for the desired synthesis. The effort to increase the loading by increasing the contact time to 2 days at a temperature of about 50 °C was successful. The copper coordinated dendritic polymer appeared as a dark green powder. It is worth mentioning that the G0, G1 and G2 catalysts were all highly functionalized, non-hygroscopic, and stable, and they can be stored for a prolonged period of time without any change in their catalytic efficiency.
 | ||
| Fig. 6 Merrifield resin supported dendrigraft GLR-G2-Cu polymer with a glycerol initiated PECH core. | ||
 | ||
| Scheme 2 Synthesis of Merrifield resin supported dendrigraft GLR-G2-Cu polymer with a glycerol initiated PECH core. | ||
3.1 Catalyst characterization
The synthesized catalyst was characterized with different techniques that are discussed as follows.Energy dispersive X-ray spectroscopic (EDX) analysis, which provides in situ chemical analysis of the bulk, was carried out by focusing on multiple regions over the surface of the polymer. The EDX spectra clearly showed Cu, C, N and O as the constituents of the catalyst (ESI†). The results presented in Table 1 are averages of the data from the different regions. The data obtained on the composition of the compounds from energy dispersive X-ray spectroscopy were consistent with the elemental analysis values (Table 1).
| Polymer | νsym(NH) | νbend(NH) | νsym(NHCO) | νbend(C–OH) | νsym(C–N) | νsym(Cu–N) | νwag(NH) |
|---|---|---|---|---|---|---|---|
| GLR-G2 | 3469 | 1562, 1565 | 1644 | 1374 | 1003 | — | 725 |
| GLR-G2-Cu | 3456 | 1565 | 1633 | 1410 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
467 | 700 |
| Polymer | g∥ | g⊥ | gav | A∥ | A⊥ | Aav |
|---|---|---|---|---|---|---|
| GLR-G2-Cu | 2.25 | 2.05 | 2.11 | 175.2 | 13.81 | 64.73 |
I.A. Gentle et al. studied the EXAFS data for copper(II)–PAMAM solutions, and the data were fitted with acceptable parameters using a model in which primary amine, amide and tertiary amine nitrogen atoms were involved in bonding with the copper(II) ion to form five- and six-membered rings.9a,31 Thus, GLR-G2-Cu contains copper coordinated to two amine nitrogens, two amide nitrogens and one tertiary nitrogen of the amidoamine unit.
In accordance with this, the diffuse reflectance UV-Vis spectra of GLR-G2-Cu (Fig. 8) showed a broad peak in the region of 580–780 nm with maximum intensity at 680 nm, which is characteristic of five coordinated copper complexes with square pyramidal geometry. The peak may be ascribed to the absorption due to the overlapping of allowed d–d transitions in copper after coordination with dendritic ligands.
The catalyst displayed a characteristic Cu 2p3/2 singlet with a peak located at 934.3 eV. Strong satellite peaks were observed at 942, 943.2, 954.3 and 962.4 eV. The binding energy values observed are in good agreement with the available literature data for Cu ions in the 2+ oxidation state.32 The presence of Cu(II) in the supported dendritic compounds has thus been confirmed from the results of XPS analysis. The XPS results are also consistent with the paramagnetic nature of the catalysts, as evidenced by the magnetic susceptibility measurements.
3.2 Catalytic activity of resin supported dendrigraft GLR-Gn-Cu complex
Recently, copper catalyzed reactions with inexpensive and less toxic copper catalysts40 and molecular oxygen have shown wide application, with high tolerance of functional groups, in benzimidazole synthesis.41 Most of the reported catalysts were homogeneous, even though they applied green chemistry principles; some catalysts were heterogeneous, but used high temperatures or oxidants other than molecular oxygen. A comparison of different catalysts used for the reaction with similar substrates is shown in Table 4. Therefore, we have tried to combine the benefits of both heterogeneous and dendritic behaviour to address the abovementioned issues.
| Catalyst | Time | Temperature | Yieldb (%) |
|---|---|---|---|
| a Reported catalysts require high temperature conditions or long reaction times, or are not reusable.b Yield with respect to benzaldehyde. | |||
| K10TiClay27a | 2 h | 120 °C | 79 |
| INDION-190 (ref. 42a) | 4 h | 70 °C | 89 |
| CAN42b | 2 h | 50 °C | 98 |
| Ti(IV)isopropoxide42c | 2 h | 100 °C | 92 |
| Glycerol–H2O42d | 2 h | 90 °C | 75 |
| Thiamine hydrochloride39a | 1.5 h | RT | 93 |
| CoO(II)42e | 6 h | RT | 93 |
| Co(OH)2 (ref. 42e) | 4 h | RT | 96 |
| FeCl3/PANI42f | 30 min | RT | 90 |
In a survey of catalytic activity, the dendrigraft GLR-Gn (n = 0, 1 and 2) copper complexes were employed in the synthesis of benzimidazole derivatives. A variety of benzimidazole derivatives have been synthesized in excellent yields from aromatic aldehydes and aliphatic or cyclic ketones (Scheme 3). To optimize the reaction conditions, the effect of various reaction parameters, such as the type of solvent, reaction temperature, substrate ratio, and catalyst concentration, were evaluated using benzaldehyde/cyclohexanone and o-phenylenediamine as model substrates and GLR–G2–Cu as the catalyst. The emphasis in the present study has been on conducting the reactions using environmentally safe solvents, including water, and to avoid the use of chlorinated solvents. Nevertheless, apart from water, methanol, ethanol, acetonitrile and DMF, we also screened the reaction in dichloromethane.
The nature of the solvent was observed to have a substantial effect on the activity of the catalyst and the product selectivity of the reaction. The reactions were performed at ambient temperature under magnetic stirring. From the results presented in Table 5, it is evident that the reaction conducted in the molar ratio of o-phenylenediamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanone/benzaldehyde of 1.0
cyclohexanone/benzaldehyde of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2/1.0 in ethanol at room temperature proceeded smoothly to selectively yield the corresponding 2,2-disubstituted or 2-substituted benzimidazole as the exclusive product.
1.2/1.0 in ethanol at room temperature proceeded smoothly to selectively yield the corresponding 2,2-disubstituted or 2-substituted benzimidazole as the exclusive product.
| Solvent | Yieldb (%) | Yieldc (%) |
|---|---|---|
| a Reaction conditions: o-phenylenediamine (1.0 mmol), cyclohexanone (1.2 mmol), benzaldehyde (1.0 mmol), catalyst (20 mg), room temperature (30 °C), air.b Isolated yield using cyclohexanone.c Isolated yield using benzaldehyde. | ||
| DMF | 22 | 20 |
| CH3CN | 35 | 23 |
| Dichloromethane | 14 | 12 |
| Ethanol | 96 | 94 |
| Methanol | 90 | 89 |
| Water | 74 | 72 |
Increasing the catalyst amount reduces the reaction time from 80 min (with 0.004 mol% catalyst) to 30 min and 60 min (with 0.007 mol% catalyst) for cyclohexanone and benzaldehyde, respectively (Table 6). Even though 0.014 mol% catalyst requires only 40 minutes for the completion of the reaction in the case of benzaldehyde, we preferred 0.007 mol% as the optimum catalyst amount. Moreover, water as the solvent gave yields of 74% and 72% for the cyclohexanone and benzaldehyde substrates, respectively; the yields were greater in ethanol, which was found to be the best solvent.
| Amount of catalyst (mg) | Amount of catalyst (mol%) | Timeb (min) | Yieldc (%) | Timed (min) | Yielde (%) |
|---|---|---|---|---|---|
| a Reaction conditions: o-phenylenediamine (1.0 mmol), cyclohexanone (1.2 mmol), benzaldehyde (1.0 mmol), ethanol (3 mL), room temperature (30 °C), air.b Time.c Isolated yield using cyclohexanone.d Time.e Isolated yield using benzaldehyde. | |||||
| 5 | 0.004 | 80 | 90 | 80 | 87 |
| 10 | 0.007 | 30 | 96 | 60 | 94 |
| 15 | 0.011 | 30 | 96 | 50 | 94 |
| 20 | 0.014 | 30 | 96 | 40 | 94 |
| 25 | 0.018 | — | — | 40 | 94 |
| 30 | 0.021 | — | — | 40 | 94 |
Furthermore, we have studied the effect of the generation of the catalyst on the synthesis of benzimidazole derivatives with benzaldehyde/cyclohexanone as substrates using 0.007 mol% catalyst (Table 7). In both cases, the G0, G1 and G2 dendrigraft copper catalysts showed good to excellent product yields. Therefore, all generations (GLR-Gn-Cu, n = 0, 1 and 2), including the low generation GLR-G0-Cu, were active catalysts towards the synthesis of benzimidazole derivatives. The negative dendritic effect was not pronounced here and the usual concept of the slow reaction of supported catalysts was also not observed.
| Benzimidazole derivative from | % yield | ||
|---|---|---|---|
| GLR-G0-Cu | GLR-G1-Cu | GLR-G2-Cu | |
| a Reaction conditions: o-phenylenediamine (1 mmol), benzaldehyde (1.0 mmol)/cyclohexanone (1.2 mmol), ethanol (3 mL), room temperature (30 °C), catalyst (0.007 mol%). Isolated yield. | |||
| Benzaldehyde | 80 | 87 | 94 |
| Cyclohexanone | 84 | 90 | 96 |
Thus, to attain a good level of conversion, GLR-G2-Cu catalyst (0.007 mol%) in ethanol at room temperature (30 °C) in the presence of air was found to be optimal (Table 5, 6 and 7). After optimizing the reaction conditions, the scope of the different substrates was examined (Table 8). All the substrates showed completion of the reaction within a few hours. Most of the reactions of o-phenylenediamine and aromatic aldehydes resulted in good to excellent yields, irrespective of whether an electron-withdrawing or an electron-donating group was present, i.e., both electron withdrawing and electron releasing substituents showed good rates of conversion. However, the position of the substitution on the phenyl ring of benzaldehyde affects the reaction yield. Fascinatingly, chloro (Table 8, d), bromo (Table 8, e), nitro (Table 8, b), and methoxy (Table 8, c) groups at the para position furnished high yields. However, chloro (Table 8, j) and methoxy (Table 8, k) groups at the ortho position provided low yields compared with the same groups at the para position. Moreover, the chloro group at the ortho position (Table 8, j) provided a lower yield than the same at the meta position (Table 8, i). To check whether the reaction is possible with aliphatic aldehydes, acetaldehyde was chosen and furnished with high yield (98% in 1 hour).
With the optimum reaction conditions in hand, we also investigated the scope of the dendritic copper-catalyzed conjugation of o-phenylenediamine with aliphatic, aromatic and cyclic ketones. As shown in Table 9, the examined substrates provided good to excellent yields. For ketones, the reactivity depended on their steric and electronic effects; in the case of cyclic ketones, particularly, there is substantial angle strain, and the addition of a nucleophile to these carbonyl bonds creates a tetrahedral center with less strain in the ring. 6-Membered rings have the least ring strain because the carbons in the 6-membered ring can all achieve the closest angles to the ideal sp3 bond angle. Therefore, in going from a sp2 hybridised centre to a sp3 hybridised centre, cyclohexanone becomes more stable, because the conformation of that ring favours the sp3 centre. However, cyclopentanone, cycloheptanone and cyclooctanone are somewhat further away from the sp3 bond angle and actually favour sp2 hybridisation, because their torsional strain increases when they go from being sp2 hybridised to sp3 hybridised. Acetophenone, benzophenone and benzyl methyl ketone were reluctant to undergo this conjugation; no reaction was observed, even after 6 h of reaction time. The higher charge density on the carbonyl carbon and the greater steric hindrance of these ketone substrates are the reasons for their lack of reactivity. In the case of dialkyl ketones, the reactivity was found to decrease as the chain length increased. In the case of isopropyl methyl ketone, the reactivity was low due to the bulky isopropyl group. The vital role of the catalyst, leading to the formation of the desired product, was confirmed by conducting a blank experiment without the catalyst. In the absence of the catalyst, the reaction was sluggish and gave only a trace amount of the product. We have further compared the heterogeneous reaction with the reported homogeneous copper acetate catalyst (Table 9),40a and the result shows that the dendritic effect enhances the catalytic behaviour to a certain extent.
| Substrate | No. of cycles | Catalyst weight (mg) | Catalyst recovered (mg) | Recovery (%) | Product yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: o-phenylenediamine (1.0 mmol), cyclohexanone (1.2 mmol, reaction time: 30 min), benzaldehyde (1.0 mmol, reaction time: 1 h), ethanol (3 mL), RT (30 °C).b Isolated yield. | |||||
| Cyclohexanone | 1 | 10 | 9.9 | 99.0 | 96 |
| 2 | 9.9 | 9.8 | 99.0 | 96 | |
| 3 | 9.8 | 9.6 | 98.0 | 94 | |
| 4 | 9.6 | 9.5 | 99.0 | 94 | |
| 5 | 9.5 | 9.3 | 97.9 | 93 | |
| Benzaldehyde | 1 | 10 | 9.9 | 99.0 | 94 |
| 2 | 9.9 | 9.7 | 98.0 | 94 | |
| 3 | 9.7 | 9.6 | 99.0 | 94 | |
| 4 | 9.6 | 9.4 | 97.9 | 93 | |
| 5 | 9.4 | 9.2 | 97.9 | 92 | |
4. Conclusions
Polyepichlorohydrin was coupled to Merrifield resin to increase the loading capacity. Novel families of dendrigraft G0, G1 and G2 amine polymers with glycerol initiated polyepichlorohydrin cores have been synthesized and characterized. The amount of amino groups in the highly functionalized G0, G1 and G2 series was found to increase from the G0 to the G2 generation. The copper complexes of these dendrigraft polymers were prepared. Characterization of the GLR-G2-Cu catalyst was performed. All the generations of GLR-Gn-Cu (n = 0, 1 and 2) were found to be excellent catalysts for the synthesis of benzimidazole derivatives via the reaction between o-phenylenediamine with aldehydes or aliphatic or cyclic ketones. The reaction occurred even with low generation catalysts, i.e., GLR-G0-Cu polymer. A detailed study of the synthesis of benzimidazole derivatives was performed with the GLR-G2-copper catalyst. The GLR-G2-Cu catalyst was also compared with nondendritic copper acetate for the synthesis of 2,2-disubstituted benzimidazoles. The main features of the synthesis include: (1) air was used as the terminal oxidant. (2) Even though the reaction proceeded well in water, ethanol was used as the solvent for the reaction. (3) Only a small amount of catalyst was needed to drive the reaction (0.007 mol%). (4) Water was the only by-product in this reaction. (5) All the reactions were performed at room temperature (30 °C) and showed outstanding tolerance of the functional groups on the aldehydes. (6) The synthetic protocol for benzimidazole derivatives is straightforward, safe, environmentally clean, and free from halogenated solvents or any other additives such as co-catalyst or acid. (7) The procedural simplicity, simple recovery and reusability of the catalysts meet the requirements of benign chemistry. Further investigation of the application of this catalyst for the synthesis of benzimidazoles using substituted aromatic ketones or carbaldehydes is in progress.Acknowledgements
We would like to thank STIC, CUSAT for assistance with various analysis. One of the authors (G.S.) thanks the CSIR, Govt. of India for financial assistance in the form of fellowship. The authors thank PSRT, CUSAT for GPC analysis, NCL, Pune for solid state 13C NMR analysis, AIIMS, Kochi for XPS, IIT, Chennai for magnetic susceptibility and IIT, Mumbai for EPR analysis. Finally we thank Thermax, India for providing the gift sample of Merrifield resin.References
-
(a) J. Yan, W. Li and A. Zhang, Chem. Commun., 2014, 50, 12221 RSC
; (b) H.-J. Sun, S. Zhang and V. Percec, Chem. Soc. Rev., 2015, 44, 3900 RSC
; (c) C. Park, J. Lee and C. Kim, Chem. Commun., 2011, 47, 12042 RSC
; (d) T. Nokami, T. Watanabe, N. Musya, T. Morofuji, K. Tahara, Y. Tobe and J.-I. Yoshida, Chem. Commun., 2011, 47, 5575 RSC
; (e) O. V. Borisov, A. A. Polotsky, O. V. Rud, E. B. Zhulina, F. A. M. Leermakerse and T. M. Birshtein, Soft Matter, 2014, 10, 2093 RSC
; (f) B. Zhang and A. D. Schlueter, New J. Chem., 2012, 36, 414 RSC
; (g) A. Carlmark, E. Malmstrom and M. Malkoch, Chem. Soc. Rev., 2013, 42, 5858 RSC
; (h) Y. Chen and X. Xiong, Chem. Commun., 2010, 46, 5049 RSC
.
-
(a) M. Gauthier, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3803 CrossRef CAS
; (b) S. J. Teerstra and M. Gauthier, Prog. Polym. Sci., 2004, 29, 277 CrossRef
.
- D. A. Tomalia, D. M. Hedstrand and M. S. Ferritto, Macromolecules, 1991, 24, 1435 CrossRef CAS
.
-
(a) H. Frauenrath, Prog. Polym. Sci., 2005, 30, 325 CrossRef CAS
; (b) A. D. Schluter, Top. Curr. Chem., 1998, 197, 165 CrossRef CAS
; (c) A. D. Schluter and J. P. Rabe, Angew. Chem., Int. Ed., 2000, 39, 864 CrossRef CAS
.
- M. Gauthier and M. Moller, Macromolecules, 1991, 24, 4548 CrossRef CAS
.
-
(a) C. Schuell and H. Frey, ACS Macro Lett., 2012, 1, 461 CrossRef CAS
; (b) H. Yu, A. D. Schlueter and B. Zhang, Macromolecules, 2014, 47, 4127 CrossRef CAS
; (c) H. Yu, A. D. Schlueter and B. Zhang, Macromolecules, 2012, 45, 8555 CrossRef CAS
; (d) E.-H. Kang, I. S. Lee and T.-L. Choi, J. Am. Chem. Soc., 2011, 133, 11904 CrossRef CAS PubMed
; (e) T. Terashima, T. Mes, T. F. A. de Greef, M. A. J. Gillissen, P. Besenius, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 4742 CrossRef CAS PubMed
.
-
(a) B. A. Laurent and S. M. Grayson, J. Am. Chem. Soc., 2011, 133, 13421 CrossRef CAS PubMed
; (b) X. Feng, D. Taton, E. Ibarboure, E. L. Chaikof and Y. Gnanou, J. Am. Chem. Soc., 2008, 130, 11662 CrossRef CAS PubMed
.
-
(a) A. Dahan and M. Portnoy, J. Am. Chem. Soc., 2007, 129, 5860 CrossRef CAS PubMed
; (b) R. C. D. Brown, J. Chem. Soc., Perkin Trans. 1, 1998, 3293 RSC
; (c) V. Swali, N. J. Wells, G. J. Langley and M. Bradley, J. Org. Chem., 1997, 62, 4902 CrossRef CAS
; (d) T. Kehat, K. Goren and M. Portnoy, New J. Chem., 2007, 31, 1218 RSC
; (e) A. Y.-T. Huang, C.-H. Tsai, H.-Y. Chen, H.-T. Chen, C.-Y. Lu, Y.-T. Lin and C.-L. Kao, Chem. Commun., 2013, 49, 5784 RSC
; (f) P. Bharathi and J. S. Moore, J. Am. Chem. Soc., 1997, 119, 3391 CrossRef CAS
; (g) S. M. Lu and H. Alper, J. Am. Chem. Soc., 2003, 125, 13126 CrossRef CAS PubMed
.
-
(a) M. F. Ottaviani, S. Bossmann, N. J. Turro and D. A. Tomalia, J. Am. Chem. Soc., 1994, 116, 661 CrossRef CAS
; (b) L. Zhou, D. H. Russell, M. Q. Zhao and R. M. Crooks, Macromolecules, 2001, 34, 3567 CrossRef CAS
; (c) M. F. Ottaviani, M. Cangiotti, A. Fattori, C. Coppola, P. Posocco, E. Laurini, X. Liu, C. Liu, M. Fermeglia, L. Peng and S. Pricl, Phys. Chem. Chem. Phys., 2014, 16, 685 RSC
; (d) Y. Wang, Q. Zhao, H. Zhang, S. Yang and X. Jia, Adv. Mater., 2014, 26, 4163 CrossRef CAS PubMed
; (e) D. Wang, C. Deraedt, L. Salmon, C. Labrugere, L. Etienne, J. Ruiz and D. Astruc, Chem.–Eur. J., 2015, 21, 1508 CrossRef CAS PubMed
; (f) C. Deraedt, D. Wang, L. Salmon, L. Etienne, C. Labrugere, J. Ruiz and D. Astruc, ChemCatChem, 2015, 7, 303 CrossRef CAS
.
-
(a) D. Astruc, Nat. Chem., 2012, 4, 255 CrossRef CAS PubMed
; (b) L. W. Hoffman, G. G. Andersson, A. Sharma, S. R. Clarke and N. H. Voelcker, Langmuir, 2011, 27, 6759 CrossRef CAS PubMed
; (c) G. R. Krishnan and K. Sreekurnar, Appl. Catal., A, 2009, 353, 80 CrossRef CAS
; (d) G. R. Krishnan and K. Sreekumar, Eur. J. Org. Chem., 2008, 4763 CrossRef CAS
.
-
(a) G. S. Smith and S. F. Mapolie, J. Mol. Catal. A: Chem., 2004, 213, 187 CrossRef CAS
; (b) R. Malgas, S. F. Mapolie, S. O. Ojwach, G. S. Smith and J. Darkwa, Catal. Commun., 2008, 9, 1612 CrossRef CAS
; (c) P. Govender, N. C. Antonels, J. Mattsson, A. K. Renfrew, P. J. Dyson, J. R. Moss, B. Therrien and G. S. Smith, J. Organomet. Chem., 2009, 694, 3470 CrossRef CAS
; (d) D. E. Bergbreiter, J. Tian and C. Hongfa, Chem. Rev., 2009, 109, 530 CrossRef CAS PubMed
; (e) N. C. Antonels, J. R. Moss and G. S. Smith, J. Organomet. Chem., 2011, 696, 2003 CrossRef CAS
; (f) R. K. G. Panicker and S. Krishnapillai, Tetrahedron Lett., 2014, 55, 2352 CrossRef
.
-
(a) S. C. Bourque, H. Alper, L. E. Manzer and P. Arya, J. Am. Chem. Soc., 2000, 122, 956 CrossRef CAS
; (b) M. A. Hearshaw and J. R. Moss, Chem. Commun., 1999, 1 RSC
; (c) D. de Groot, P. G. Emmerink, C. Coucke, J. N. H. Reek, P. C. J. Kamer and P. van Leeuwen, Inorg. Chem. Commun., 2000, 3, 711 CrossRef CAS
; (d) B. Blom, M. J. Overett, R. Meijboom and J. R. Moss, Inorg. Chim. Acta, 2005, 358, 3491 CrossRef CAS
; (e) T. Fujihara, Y. Obora, M. Tokunaga, H. Sato and Y. Tsuji, Chem. Commun., 2005, 4526 RSC
; (f) A. Perrier, M. Keller, A.-M. Caminade, J.-P. Majoral and A. Ouali, Green Chem., 2013, 15, 2075 RSC
; (g) M. Keller, A. Hameau, G. Spataro, S. Ladeira, A.-M. Caminade, J.-P. Majoral and A. Ouali, Green Chem., 2012, 14, 2807 RSC
.
-
(a) G. R. Krishnan and K. Sreekumar, Polymer, 2008, 49, 5233 CrossRef CAS
; (b) S. M. Lu and H. Alper, J. Am. Chem. Soc., 2005, 127, 14776 CrossRef CAS PubMed
; (c) Y. M. Chung and H. K. Rhee, Chem. Commun., 2002, 238 RSC
.
-
(a) D. Dai, J. R. Burgeson, D. N. Gharaibeh, A. L. Moore, R. A. Larson, N. R. Cerruti, S. M. Amberg, T. C. Bolken and D. E. Hruby, Bioorg. Med. Chem. Lett., 2013, 23, 744 CrossRef CAS PubMed
; (b) A. Husain, M. Rashid, M. Shaharyar, A. A. Siddiqui and R. Mishra, Eur. J. Med. Chem., 2013, 62, 785 CrossRef CAS PubMed
; (c) J. E. Payne, C. Bonnefous, K. T. Symons, P. M. Nguyen, M. Sablad, N. Rozenkrants, Y. Zhang, L. Wang, N. Yazdani, A. K. Shiau, S. A. Noble, P. Rix, T. S. Rao, C. A. Hassig and N. D. Smith, J. Med. Chem., 2010, 53, 7739 CrossRef CAS PubMed
.
- R. J. Cherney, R. Mo, D. T. Meyer, A. D. Pechulis, M. A. Guaciaro, Y. C. Lo, G. Yang, P. B. Miller, P. A. Scherle, Q. Zhao, M. E. Cvijic, J. C. Barrish, C. P. Decicco and P. H. Carter, Bioorg. Med. Chem. Lett., 2012, 22, 6181 CrossRef CAS PubMed
.
- W. Ginzinger, G. Muehlgassner, V. B. Arion, M. A. Jakupec, A. Roller, M. Galanski, M. Reithofer, W. Berger and B. K. Keppler, J. Med. Chem., 2012, 55, 3398 CrossRef CAS PubMed
.
- S.-C. Tsay, J. R. Hwu, R. Singha, W.-C. Huang, Y. H. Chang, M.-H. Hsu, F.-k. Shieh, C.-C. Lin, K. C. Hwang, J.-C. Horng, E. de Clercq, I. Vliegen and J. Neyts, Eur. J. Med. Chem., 2013, 63, 290 CrossRef CAS PubMed
.
-
(a) S.-C. Lee, D. Shin, J. M. Cho, S. Ro and Y.-G. Suh, Bioorg. Med. Chem. Lett., 2012, 22, 1891 CrossRef CAS PubMed
; (b) J. M. Travins, R. C. Bernotas, D. H. Kaufman, E. Quinet, P. Nambi, I. Feingold, C. Huselton, A. Wilhelmsson, A. Goos-Nilsson and J. Wrobel, Bioorg. Med. Chem. Lett., 2010, 20, 526 CrossRef CAS PubMed
; (c) Y. V. Tomilov, D. N. Platonov, A. E. Frumkin, D. L. Lipilin and R. F. Salikov, Tetrahedron Lett., 2010, 51, 5120 CrossRef CAS
.
-
(a) V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12696 CrossRef CAS PubMed
; (b) V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. G. Finn, J. Am. Chem. Soc., 2013, 135, 1626 CrossRef CAS
; (c) M. Kose and V. McKee, Polyhedron, 2014, 75, 30 CrossRef CAS
.
-
(a) S. Song, Y. Jin, S. H. Park, S. Cho, I. Kim, K. Lee, A. J. Heeger and H. Suh, J. Mater. Chem., 2010, 20, 6517 RSC
; (b) J. Kim, S. H. Park, J. Kim, S. Cho, Y. Jin, J. Y. Shim, H. Shin, S. Kwon, I. Kim, K. Lee, A. J. Heeger and H. Suh, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 369 CrossRef CAS
; (c) A. C. Ozelcaglayan, M. Sendur, N. Akbasoglu, D. H. Apaydin, A. Cirpan and L. Toppare, Electrochim. Acta, 2012, 67, 224 CrossRef CAS
; (d) B. Yu, H. Zhang, Y. Zhao, S. Chen, J. Xu, C. Huang and Z. Liu, Green Chem., 2013, 15, 95 RSC
; (e) R. Cano, D. J. Ramon and M. Yus, J. Org. Chem., 2011, 76, 654 CrossRef CAS PubMed
; (f) K. Osowska and O. S. Miljanic, J. Am. Chem. Soc., 2011, 133, 724 CrossRef CAS PubMed
; (g) L. Hao, Green Chem., 2014, 16, 3039 RSC
.
-
(a) B. Gaur, B. Lochab, V. Choudhary and I. K. Varma, J. Macromol. Sci., Polym. Rev., 2003, C43, 505 CrossRef CAS
; (b) T. Biedron, P. Kubisa and S. Penczek, J. Polym. Sci., Part A: Polym. Chem., 1991, 29, 619 CrossRef CAS
.
-
(a) O. W. Gooding, S. Baudart, T. L. Deegan, K. Heisler, J. W. Labadie, W. S. Newcomb, J. A. Porco and P. van Eikeren, J. Comb. Chem., 1999, 1, 113 CrossRef CAS
; (b) J. Karabline and M. Portnoy, Org. Biomol. Chem., 2012, 10, 4788 RSC
; (c) R. R. Karimov, Z.-G. M. Kazhkenov, M. J. Modjewski, E. M. Peterson and V. V. Zhdankin, J. Org. Chem., 2007, 72, 8149 CrossRef CAS PubMed
.
- G. Ampleman, US Pat., 5, 124, 463, 1992
.
- R. I. Wagner, US Pat., EP0380750, 1989
.
-
(a) M. Dardouri, F. Ammari and F. Meganem, Int. J. Polym. Sci., 2015, 2015, 280325 Search PubMed
; (b) X. G. Han, R. A. Shanks and D. Pavel, Eur. Polym. J., 2005, 41, 984 CrossRef CAS
.
- I. J. Majoros, C. R. Williams, D. A. Tomalia and J. R. Baker Jr, Macromolecules, 2008, 41, 8372 CrossRef CAS PubMed
.
-
(a) V. Kannan and K. Sreekumar, J. Mol. Catal. A: Chem., 2013, 376, 34 CrossRef CAS
; (b) K. Mangala and K. Sreekumar, J. Appl. Polym. Sci., 2013, 127, 717 CrossRef CAS
; (c) K. Mangala and K. Sreekumar, J. Appl. Polym. Sci., 2015, 132, 41593 CrossRef
.
- D. Wang and D. Astruc, Coord. Chem. Rev., 2013, 257, 2317 CrossRef CAS
.
- K. Nakamoto, Infrared and Ramam spectra of Inorganic and Co-ordination Compounds, Part B, Wiley and Sons, New York, 5th edn, 1997 Search PubMed
.
-
(a) P. R. S. Thakurta, G. Rosair, C. J. Gomez-Garcia, E. Garribba and S. Mitra, Polyhedron, 2009, 28, 695 CrossRef
; (b) V. P. Singh, Spectrochim. Acta, Part A, 2008, 71, 17 CrossRef PubMed
.
- M. L. Tran, L. R. Gahan and I. R. Gentle, J. Phys. Chem. B, 2004, 108, 20130 CrossRef CAS
.
-
(a) M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887 CrossRef CAS
; (b) D. Briggs, Practical Surface Analysis, Auger and X-ray Photoelectron Spectroscopy, Wiley, 1996, vol. 1 Search PubMed
.
-
(a) W. Mo, H. Liu, H. Xiong, M. Li and G. Li, Appl. Catal., A, 2007, 333, 172 CrossRef CAS
; (b) G. Lisa, E. Avram, G. Paduraru, M. Irimia, N. Hurduc and N. Aelenei, Polym. Degrad. Stab., 2003, 82, 73 CrossRef CAS
.
-
(a) K. Selim, S. Ozkar and L. Yilmaz, J. Appl. Polym. Sci., 2000, 77, 538 CrossRef CAS
; (b) S. K. Sahu, S. P. Panda, D. S. Sadafule, C. G. Kumbhar, S. G. Kulkarni and J. V. Thakur, Polym. Degrad. Stab., 1998, 62, 495 CrossRef CAS
.
-
(a) M. R. Grimmet, Comprehensive Heterocyclic Chemistry, Pergamon Press, Oxford, 1984 Search PubMed
; (b) J. B. Wright, Chem. Rev., 1951, 48, 396 CrossRef
; (c) R. W. Middleton, J. Heterocycl. Chem., 1980, 17, 1757 CrossRef CAS
; (d) T. Hisano, Chem. Pharm. Bull., 1982, 30, 2996 CrossRef CAS
; (e) J. D. Geratz, Arch. Biochem. Biophys., 1979, 197, 551 CrossRef CAS PubMed
.
-
(a) A. Czarny, W. D. Wilson and D. W. Boykin, J. Heterocycl. Chem., 1996, 33, 1393 CrossRef CAS
; (b) R. R. Tidwell, J. Med. Chem., 1978, 21, 613 CrossRef CAS PubMed
; (c) T. A. Fairley, R. R. Tidwell, I. Donkor, N. A. Naiman, K. A. Ohemeng, R. J. Lombardy, J. A. Bentley and M. Cory, J. Med. Chem., 1993, 36, 1746 CrossRef CAS PubMed
.
-
(a) K. Bourgrin, Tetrahedron, 1998, 54, 8055 CrossRef
; (b) G. V. Reddy, V. Rao, B. Narsaiah and P. S. Rao, Synth. Commun., 2002, 32, 2467 CrossRef CAS
; (c) A. Ben-Alloum, S. Bakkas and M. Soufiaoui, Tetrahedron Lett., 1998, 39, 4481 CrossRef CAS
.
-
(a) S. Song, H. Han, Y. Kim, B. H. Lee, S. H. Park, Y. Jin, I. Kim, K. Lee and H. Suh, Sol. Energy Mater. Sol. Cells, 2011, 95, 1838 CrossRef CAS
; (b) S. Song, J. Kim, J. Y. Shim, G. Kim, B. H. Lee, Y. Jin, S. H. Park, I. Kim, K. Lee and H. Suh, Synth. Met., 2012, 162, 988 CrossRef CAS
; (c) P. Ghosh and R. Subba, Tetrahedron Lett., 2015, 56, 2691 CrossRef CAS
; (d) S. Hati, G. K. Patra, J. P. Naskar, M. G. B. Drew and D. Datta, New J. Chem., 2001, 25, 218 RSC
.
-
(a) M. Lei, L. Ma and L. Hu, Synth. Commun., 2012, 42, 2981 CrossRef CAS
; (b) J. Yuan, Z. Zhao, W. Zhu, H. Li, X. Qian and Y. Xu, Tetrahedron, 2013, 69, 7026 CrossRef CAS
; (c) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, J. Org. Chem., 2011, 76, 8394 CrossRef CAS PubMed
; (d) P. Saha, M. A. Ali, P. Ghosh and T. Punniyamurthy, Org. Biomol. Chem., 2010, 8, 5692 RSC
; (e) H. Sharma, N. Singh and D. O. Jang, Green Chem., 2014, 16, 4922 RSC
.
-
(a) J. Lu, H. Yang, Y. Jin, Y. Jiang and H. Fu, Green Chem., 2013, 15, 3184 RSC
; (b) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS PubMed
; (c) C. Chen, J. Org. Chem., 2011, 76, 716 CrossRef PubMed
; (d) Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC
; (e) A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef CAS PubMed
; (f) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed
.
-
(a) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13 RSC
; (b) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS PubMed
; (c) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed
; (d) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS PubMed
; (e) H. Rao, Synlett, 2011, 745 CAS
; (f) T. Liu and H. Fu, Synthesis-Stuttgart, 2012, 44, 2805 CrossRef CAS
.
-
(a) V. S. Padalkar, V. D. Gupta and N. Sekar, Green Chem. Lett. Rev., 2012, 5, 139 CrossRef CAS
; (b) M. Kidwai, A. Jahan and D. Bhatnagar, J. Chem. Sci., 2010, 122, 607 CrossRef CAS
; (c) F. H. Havaldar, G. Mule and B. Dabholkar, Synth. Commun., 2011, 41, 2304 CrossRef CAS
; (d) H. M. Bachhav, S. B. Bhagat and V. N. Telvekar, Tetrahedron Lett., 2011, 52, 5697 CrossRef CAS
; (e) M. A. Chari, D. Shobha and T. Sasaki, Tetrahedron Lett., 2011, 52, 5575 CrossRef
; (f) M. Abdollahi-Alibeik and M. Moosavifard, Synth. Commun., 2010, 40, 2686 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: GPC profile, IR, solid state 13C NMR and EDX spectrum. XRD, XPS, EPR and TG-DTG profile. Bar diagram of reusability of catalyst. Product data of benzimidazole derivatives. See DOI: 10.1039/c5ra28046j |
| This journal is © The Royal Society of Chemistry 2016 |