Highly uniform indicator-mediated SERS sensor platform for the detection of Zn2+†
Liangliang Jinab,
Guangwei She*a,
Lixuan Mua and
Wensheng Shia
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: shegw@mail.ipc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 29th January 2016
Abstract
An indicator-mediated surface-enhanced Raman scattering sensor platform with highly uniform SERS sensitivity was fabricated by modifying 4-mercaptopyridine molecules onto the surface of Ag nanoparticles that were anchored onto a silicon wafer. The present SERS sensor platform realized quantitative detection of Zn2+.
1. Introduction
Zinc ion (Zn2+) has attracted a great deal of attention ascribed to its biological significance. Zn2+ is believed to be involved in some pathological processes, such as Alzheimer's disease, epilepsy, ischemic stroke, and infantile diarrhea.1–3 Some methodologies have been established to determine Zn2+ over the last decades, including atomic absorption spectrophotometry, fluorescence spectroscopy, mass spectrometry and electrochemical analysis.3–6 However, these techniques require extensive sample preparation.7–11 So, it is required to develop a facile technique for the determination of Zn2+.Surface-enhanced Raman scattering (SERS) as a prospective analytical method has attracted more and more attentions owing to its advantages such as low cost, high selectivity, ultrasensitivity, long-term stability and good compatibility with aqueous environments.12–15 However, as a molecular spectroscopy technique, SERS could only reveal the vibrational, rotational and other low-frequency modes in a system. In principle, the SERS technique cannot be directly utilized to analyze the atomic ions, which are lack of the vibrational signatures.12,16 In order to use the SERS to detect the atomic ions, a so-called indicator-mediated SERS strategy was developed. In this strategy, molecule indicators are modified onto the plasmonic surfaces of metal.16–20 When the indicator-mediated SERS sensor is used to detect the atomic ions, the interaction between the molecule indicator and atomic ions could influence the Raman band intensity and shift of the indicator. Such variations of Raman spectroscopy could be utilized as the spectral characteristics for the detection of atomic ions.21 Chen et al. used the variation of the Raman band intensity of molecule indicator functionalized silver nanoparticles for the highly sensitive detection of Hg2+ and As3+.22,23 The variation of the Raman band shift was also used for the detection of some ions.24–26 The indicator-mediated SERS sensor based on colloidal nanoparticles had been used for the detection of Zn2+.17 However, this colloidal nanoparticles-based sensor is not adequate for quantitative detection and the real time and in situ monitoring of Zn2+. A highly uniform plasmonic platform is more promising for the application of the indicator-mediated SERS strategy. Moreover, in the previously reported SERS sensor, 4-(2-pyridylazo) resorcinol and 1-(2-pyridylazo)-2-naphthol have been used as colorimetric reagent for Zn2+ since they can form very stable and highly colored complexes with Zn2+.17,27 However, the similar Raman spectra of the complexes make it difficult to distinguish the Zn2+ from the other ions. In order to promote the selectivity of the indicator-mediated SERS sensor for Zn2+, an appropriate molecule indicator owning specific interaction with Zn2+ should be developed. Such molecule indicator should have distinct Raman characteristics, and could be efficiently and rationally decorated onto the plasmonic surfaces of metals. The interaction between molecule indicator and target Zn2+ should also be investigated to promote the indicator-mediated SERS strategy. Accordingly, selecting a special molecule indicator, taking insight into the interaction between molecule indicator and target Zn2+ and developing a highly uniform plasmonic platform for detecting Zn2+ would be a key issue to develop high performance indicator-mediated SERS sensor for the detection of Zn2+.
In this study, 4-mercaptopyridine (4-MPY) was chosen as the special molecule indicator to be modified onto the surface of the Ag nanoparticles, and a highly uniform indicator-mediated SERS sensor platform for Zn2+ was fabricated. The SERS sensor platform can distinguish Zn2+ from various common metal ions. Especially, it can distinguish Zn2+ from Cd2+ that is usually difficult for fluorescent sensor and SERS sensor.9,18 Quantitative detection of Zn2+ in aqueous solution is realized by employing the linear dependence of area ratios between two distinctive Raman bands (the shifted Raman band 1027 cm−1 and the unshifted band 1102 cm−1 of 4-MPY) on the logarithmic concentrations of Zn2+. Moreover, the interaction between Zn2+ and 4-MPY had been investigated by the means of Raman, FTIR and XPS. The current work represents an effective technique for detection of Zn2+ in aqueous solution, which is prospective for life sciences.
2. Experimental details
2.1 SERS-active substrate preparation
The Ag nanoparticles-anchored Si (AgNP–Si) substrate was prepared by immersing the cleaned Si wafer into an aqueous solution consisting of 5 mM AgNO3 and 4.8 M HF for 10 seconds. After rinsing thoroughly with deionized water, the AgNP–Si substrate was soaked in the 1 mM 4-MPY ethanol solution for 1 hour to assembling 4-MPY molecules onto the surface of AgNPs. Then the substrate was washed with ethanol to remove the physically adsorbed 4-MPY molecules and the SERS-active substrate was obtained.2.2 Metal ions detection
The as-prepared SERS-active substrates were immersed respectively in aqueous solutions of metal chlorides containing various metal ions, including Zn2+, Mn2+, Ca2+, K+, Ni2+, Fe2+, Cd2+ and Cr3+. After the immersion, the substrates were rinsed with deionized water and dried in air. SERS spectra were collected from the SERS-active substrates treated with above metal ions.2.3 Instrumentation
The SERS measurements were performed using a Renishaw Raman system-inVia-Reflex with an excitation laser wavelength of 532 nm, excitation intensity of 0.5 mW, and data acquisition time of 10 s throughout these experiments. The laser beam was focused on a spot about 3 μm in diameter by a 50× microscope objective. The Raman band of a silicon wafer at 520 cm−1 was employed to calibrate the spectrometer. FTIR spectra of all the samples were recorded using Excalibur 3100 with a resolution of 4 cm−1. The morphologies of the substrates were characterized by field emission scanning electron microscopy (FE-SEM, Hitachi S-4800) and atomic force microscopy (Bruker Multimode 8) under tapping-mode. The kind of tip for AFM images is Bruker RTESPA. The X-ray photoelectron spectroscopy (XPS) was recorded by a PHIQUANTERA-IISXM at a voltage of 15 kV.3. Results and discussion
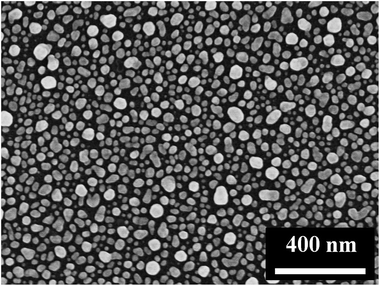
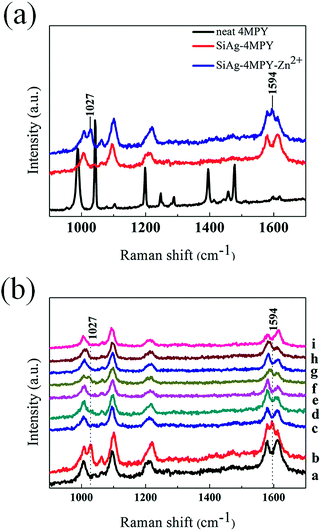
By immersing the cleaned Si wafer into an aqueous solution consisting of AgNO3 and HF for a desired time, Ag deposition could take place on the surface of the Si wafer and the Ag nanoparticles-coated Si (AgNP–Si) substrate was obtained.28 The morphology of the as-prepared AgNP–Si substrate is shown in Fig. 1 and S1.† The 2D and 3D AFM topographical images of AgNP–Si substrate are identical with the SEM images, as shown in Fig. S2.† The spherical AgNPs have an average diameter of 70 nm, which is appropriate for SERS enhancement.29The substrate was considered to be chemically modified with 4-MPY (molecule indicator) after being treated with 4-MPY ethanol solution. Fig. 2a shows the Raman spectra of the 4-MPY molecules-modified AgNP–Si substrate (SiAg–4MPY). The measured Raman spectrum of 4-MPY shows a relatively strong Raman peak intensity at the concentration of 1 mM, which is hard to be detected in aqueous solution by the means of Raman spectrum. The AgNP–Si substrate exhibits a significant enhancement on Raman signal of 4-MPY. It implies that the AgNP–Si substrate is appropriate for SERS enhancement. Moreover, to examine the uniform SERS sensitivity of the substrate, the SERS spectra of 4-MPY form 441 points on the substrate were obtained, as shown in Fig. S3a.† The relative standard deviation of the Raman counts from the 441 points at 1102 cm−1 is 6.2%, which indicates that the AgNP–Si substrate has high uniformity of SERS sensitivity. The kind of Ag-based SERS substrates could decrease SERS activity due to the adsorption of chloride ions or the formation of AgCl on the Ag surface.30 To test the SERS activity of the substrate from the Cl− attack, the SERS substrates have been treated by KCl with different concentrations for 5 hours. The results have shown that the SERS spectra of the sensor did not change even when the concentration of KCl was as high as 500 mM, as shown in Fig. S4a.†
The SiAg–4MPY was used for the detection of an important metal ion, i.e. Zn2+. After treating the SiAg–4MPY with Zn2+ solution (10 mM) (SiAg–4MPY–Zn2+), new Raman bands located at 1027 cm−1 and 1594 cm−1 were observed, as shown in Fig. 2a. The appearance of new Raman bands makes it possible to utilize the SiAg–4MPY as an indicator-mediated SERS sensor for the detection of Zn2+. To demonstrate the selectivity of the SERS sensor for Zn2+, the response of the SERS sensor to various metal ions was measured, which results are shown in Fig. 2b. By careful comparing the SERS spectra of SiAg–4MPY which are treated with 8 kinds of metal ions, Mn2+, Ca2+, K+, Ni2+, Fe2+, Cd2+ and Cr3+ do not induce the two specific Raman bands of 4-MPY as Zn2+ does. And the two new Raman bands could be employed as the spectral characteristics to selectively detect Zn2+. Furthermore, the Raman spectra response of SiAg–4MPY toward Zn2+ in the presence of the other cations was also investigated (Fig. S5†). The results showed that Mn2+, Ca2+, K+, Ni2+ and Fe2+ have ignorable influences on the detection of Zn2+, but the presence of Cd2+ and Cr3+ will inhabit the SERS sensor for detection of Zn2+. It implies that the presented SERS sensor platform can distinguish Zn2+ from the other 7 cations separately, but it can't detect Zn2+ when Cd2+ or Cr3+ is present. It should be mentioned that although the presented SERS sensor platform can't detect Zn2+ when Cd2+ is present, but it can distinguish Zn2+ from Cd2+ separately which is also an improvement for SERS sensor as the previous report obtained indistinguishable SERS spectra when detect Zn2+ and Cd2+.18 The results showed that the SERS sensor exhibited excellent selectivity for Zn2+ over various common metal ions. The anti-interference ability of the sensor should be further improved, and the follow-up optimization work aiming to improve the sensitivity and the anti-interference ability of the sensor is under way.
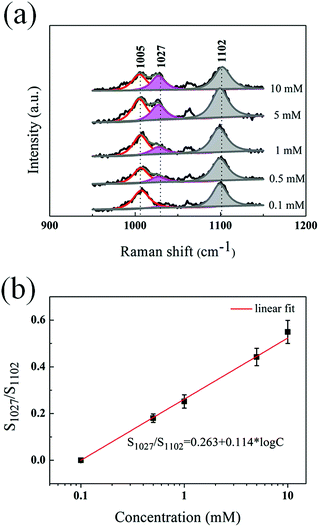
The intensities of the two new Raman bands varied with the immersing times, as shown in Fig. S6.† To obtain the quantitative information of the sensor platform, we investigate the detection of Zn2+ with concentrations range from 0.1 mM to 10 mM. For this detection, the SiAg–4MPY substrates were immersed in Zn2+ solutions for 20 hours. Fig. S7† shows that the intensity of the two new bands of 1027 cm−1 and 1594 cm−1 are enhanced with increase of the Zn2+ concentration. The quantitative information regarding the concentration of Zn2+ was obtained by comparing the band area ratios between the shifted Raman band 1027 cm−1 and the unshifted band 1102 cm−1 of 4-MPY. As the overlaps with its neighbor peak, the peak of 1027 cm−1 should be separately first. The areas under the bands 1008 cm−1 and 1027 cm−1 were deconvoluted assuming a Lorentzian shape, where the band position and the full width at half maxima were fixed (Fig. 3a).16 And the area under the band 1102 cm−1 was also obtained by fitting the band with a Lorentzian shape. Applying this process to all the spectra, the band area ratio S1027/S1102 between band 1027 cm−1 and band 1102 cm−1 can be obtained for each spectrum. We plotted the area ratios as a function of the Zn2+ concentrations. Fig. 3b reveals a linear relation between the area ratios and the logarithmic concentrations. It has been discussed in many previous reports that the SERS intensity of the molecular probe linearly increases with the logarithm of the concentration of target.31–34 It is believed that the logarithm law is related with the adsorption behaviors of Zn2+. This linear curve provides a calibration for quantitative detection of Zn2+. Moreover, the SERS spectra from the sensor indicate the high uniformity of the sensor, as shown in Fig. S3b.† The relative standard deviation of the Raman counts from the 441 points at 1027 cm−1 is 6.5%. The influence of Cl− on the SERS detection of Zn2+ was investigated by adding KCl (10–500 mM) to 10 mM ZnCl2 solution. The results shown in Fig. S4b† reveal no obvious changes in the SERS spectra of theses samples, implying that the influence of Cl− on the SERS sensor platform is negligible. The results indicate that SiAg–4MPY could be used as an effective and uniform SERS sensor platform.
To improve the detection performance of this sensor platform, we have optimized the structure of the SERS substrate by depositing more Ag nanoparticles. The immersing time for depositing Ag nanoparticles had been extended to be 60 s, 180 s and 300 s. The SEM images of as-optimized SERS substrate in Fig. S8† show that Ag nanoparticles were getting bigger and getting more. The optimized sensor platforms were used to detect Zn2+ and the results are shown in Fig. S9.† The optimized sensor platforms exhibit stronger band intensity as compared with the un-optimized platform at the same concentration of Zn2+. It implies that the sensor platform can be optimized to improve its detection performance.
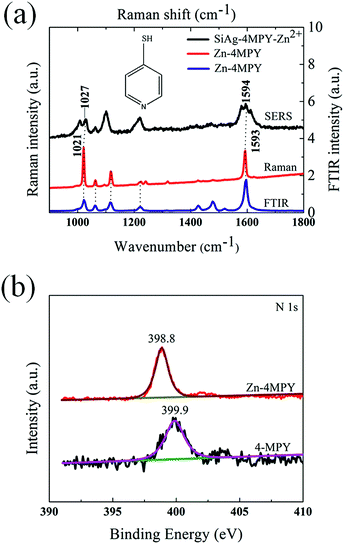
To understand the selectivity of the SERS sensor for Zn2+, it is necessary to investigate the origin of two new Raman bands in the case of Zn2+. In order to explore the origin of two new Raman bands, the Raman spectrum of the sensor response to Zn2+ (SiAg–4MPY–Zn2+) was compared with that of the composite of zinc and 4-MPY (i.e., Zn–4MPY). The Zn–4MPY was synthesized by the reaction of 4-MPY and ZnCl2 in aqueous solution with vigorous stirring. The reaction flask was wrapped with Al foil to minimize exposure to ambient light. After 1 h reaction, the resultant white precipitate (Zn–4MPY) was collected via filtration and washed with deionized water and then dried in air at room temperature. Fig. 4a shows the FT-Raman spectrum of Zn–4MPY. It has to be mentioned that, according to some reports, the organic–inorganic material might be reacted under laser illumination and thus led to the change of Raman spectrum.35 In order to clarify that the spectrum obtained in our experiment is still the Raman spectrum of Zn–4MPY, the vibrational spectroscopic technique (FTIR) was used to study Zn–4MPY, as shown in Fig. 4a. The FTIR spectrum band frequencies of Zn–4MPY are in accordance with Raman Spectrum band frequencies of Zn–4MPY shown in Fig. 2a. The FTIR spectrum reveals the intrinsic vibrational and rotational modes of Zn–4MPY. The accordance of the FTIR spectrum band frequencies and the Raman spectrum band frequencies implies that the FT-Raman spectrum obtained in Fig. 4a should be the Raman spectrum of Zn–4MPY without any change.
The FT-Raman spectrum of Zn–4MPY was compared with the SERS spectrum of SiAg–4MPY–Zn2+, as shown in Fig. 4a. The two new bands observed in the SERS spectrum of SiAg–4MPY–Zn2+ correspond well to the two most intensive bands of Zn–4MPY. It reveals that the origin of two new Raman bands for SiAg–4MPY–Zn2+ came from Zn–4MPY. The ring breathing observed at 988 cm−1 in the case of 4-MPY molecules shifted to 1021 cm−1 in the case of Zn–4MPY and 1027 cm−1 in the case of SiAg–4MPY–Zn2+. The C–C stretching observed at 1618 cm−1 in the case of 4-MPY molecules shifted to 1593 cm−1 in the case of Zn–4MPY and 1594 cm−1 in the case of SiAg–4MPY–Zn2+.19,36 The shifted ring breathing and C–C stretching implies the interaction of functional group in 4-MPY with Zn2+. 4-MPY is a typical aromatic thiol compound. It consists of a thiol group in the para position to N atom in the pyridine ring. This structure may allow it to form well-ordered self-assembled monolayers on the Ag surface through the thiol group, leaving the pyridyl nitrogen to interact with Zn2+.25,37 Moreover, the nitrogen atom contains the relatively high-energy non-bonding electron pair which makes the nitrogen atom easy to be bound by coordination of Zn2+.1 The electron donation from nitrogen orbitals to Zn2+ leads to the charge redistribution in the pyridine ring of 4-MPY and the charge redistribution results in the variation of static polarizabilitities of the ring breathing and C–C stretching. Accordingly, the Raman shifts of the two vibrational modes are observed.38
X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that can be used to study the chemical state and electronic state of the N element of 4-MPY before and after the interaction with Zn2+. Xue et al. fabricated TiO2/4-MPY/Ag sandwich structure to enhance Raman signal and investigated the interaction between 4-MPY and AgNPs by means of XPS.39 The results indicated that the Ag–S bond was more stable than the Ag–N and the AgNPs were mainly assembled through the S atom of 4-MPY.39 In regarding of this, the XPS analysis was performed mainly for the N 1s region. We have performed the experiment to investigate the N element of 4-MPY before and after the interaction with Zn2+. But unfortunately, none XPS spectrum of N 1s region was obtained for the samples. This might be due to that the very little amount of 4-MPY molecules modified on the substrate and the molecules cannot be recognized by XPS. The SERS spectrum of SiAg–4MPY–Zn2+ have the band vibration according to Zn–4MPY, as can be seen in Fig. 4a. So the interaction between 4-MPY molecule and Zn2+, which induced the Raman band accordance, is same in SiAg–4MPY–Zn2+ and Zn–4MPY. Therefore, to investigate the interaction between 4-MPY molecule and Zn2+, an XPS analysis was performed for the 4-MPY molecules and Zn–4MPY, as shown in Fig. 4b. The N 1s signal of 4-MPY molecules is centered at 399.9 eV.39,40 It is noticed that one peak located at 398.8 eV was observed for Zn–4MPY in Fig. 4b. The shift in binding energy of N 1s orbitals from 4-MPY molecules to Zn–4MPY implies that pyridyl nitrogen had interacted with Zn2+, which is accordance with the analyzed results of the as discussed Raman spectral data.
4. Conclusion
A highly uniform SERS sensitivity indicator-mediated SERS sensor platform of 4-MPY molecules modifying Ag nanoparticles based on silicon wafer (SiAg–4MPY) was proposed for the selective and quantitative detection of Zn2+. The SERS spectrum of the molecule indicator used to detect Zn2+ was characterized by features of the two new Raman bands observed at 1027 cm−1 and 1594 cm−1. Through the Raman spectra and XPS analysis, it was proved that 4-MPY molecules bind on Ag surface through the thiol group and interact with Zn2+ through the pyridyl nitrogen. It can be determined that the interaction between pyridyl nitrogen of 4-MPY molecule and Zn2+ influences the ring breathing and C–C stretching of the molecule, and results in the two new Raman bands. The indicator-mediated SERS sensor platform allows for selective and quantitative detection of Zn2+ in aqueous solution. We anticipate this kind of sensor to be of key importance in the study of environmental protection, food safety applications and life sciences.Acknowledgements
This work was supported by Chinese Academy of Sciences (Grant KGZD-EW-T02), NSFC (Grants 51272302, 51272258 and 91333119), National Basic Research Program of China (973 Program) (Grant 2012CB932400) and Youth Innovation Promotion Association CAS.Notes and references
- Z. Xu, J. Yoon and D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996–2006 RSC.
- N. Singla, A. Tripathi, M. Rana, S. Kishore Goswami, A. Pathak and P. Chowdhury, J. Lumin., 2015, 165, 46–55 CrossRef CAS.
- J. E. Kwon, S. Lee, Y. You, K. H. Baek, K. Ohkubo, J. Cho, S. Fukuzumi, I. Shin, S. Y. Park and W. Nam, Inorg. Chem., 2012, 51, 8760–8774 CrossRef CAS PubMed.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS PubMed.
- Z. Xu, K. H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601–610 CrossRef CAS PubMed.
- P. Wang, L. Liu, P. Zhou, W. Wu, J. Wu, W. Liu and Y. Tang, Biosens. Bioelectron., 2015, 72, 80–86 CrossRef CAS PubMed.
- D. L. Giokas, G. Z. Tsogas, A. G. Vlessidis and M. I. Karayannis, Anal. Chem., 2004, 76, 1302–1309 CrossRef CAS PubMed.
- Q. W. He, E. W. Miller, A. P. Wong and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9316–9317 CrossRef CAS PubMed.
- X. J. Peng, J. J. Du, J. L. Fan, J. Y. Wang, Y. K. Wu, J. Z. Zhao, S. G. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500 CrossRef CAS PubMed.
- L. Xue, C. Liu and H. Jiang, Org. Lett., 2009, 11, 1655–1658 CrossRef CAS PubMed.
- L. Ding, W. Cheng, X. J. Wang, S. J. Ding and H. X. Ju, J. Am. Chem. Soc., 2008, 130, 7224 CrossRef CAS PubMed.
- J. F. Li, Y. F. Huang, Y. Ding, Z. L. Yang, S. B. Li, X. S. Zhou, F. R. Fan, W. Zhang, Z. Y. Zhou, Y. Wu de, B. Ren, Z. L. Wang and Z. Q. Tian, Nature, 2010, 464, 392–395 CrossRef CAS PubMed.
- P. L. Stiles, J. A. Dieringer, N. C. Shah and R. P. Van Duyne, Annu. Rev. Anal. Chem., 2008, 1, 601–626 CrossRef CAS PubMed.
- J.-C. Bian, Z. Li, Z.-D. Chen, H.-Y. He, X.-W. Zhang, X. Li and G.-R. Han, Appl. Surf. Sci., 2011, 258, 1831–1835 CrossRef CAS.
- A. J. Frank, A. McEneny-King, N. Cathcart and V. Kitaev, RSC Adv., 2015, 5, 73919–73925 RSC.
- D. Tsoutsi, J. M. Montenegro, F. Dommershausen, U. Koert, L. M. Liz-Marzan, W. J. Parak and R. A. Alvarez-Puebla, ACS Nano, 2011, 5, 7539–7546 CrossRef CAS PubMed.
- L. Szabó, L. F. Leopold, B. I. Cozar, N. Leopold, K. Herman and V. Chiş, Cent. Eur. J. Chem., 2011, 9, 410–414 CrossRef.
- J. Yin, T. Wu, J. Song, Q. Zhang, S. Liu, R. Xu and H. Duan, Chem. Mater., 2011, 23, 4756–4764 CrossRef CAS.
- Y. Zhao, J. N. Newton, J. Liu and A. Wei, Langmuir, 2009, 25, 13833–13839 CrossRef CAS PubMed.
- S. J. Lee and M. Moskovits, Nano Lett., 2011, 11, 145–150 CrossRef CAS PubMed.
- R. A. Alvarez-Puebla and L. M. Liz-Marzan, Angew. Chem., 2012, 51, 11214–11223 CrossRef CAS PubMed.
- L. Chen, N. Qi, X. Wang, L. Chen, H. You and J. Li, RSC Adv., 2014, 4, 15055 RSC.
- J. Li, L. Chen, T. Lou and Y. Wang, ACS Appl. Mater. Interfaces, 2011, 3, 3936–3941 CAS.
- Z. Jurasekova, A. Torreggiani, M. Tamba, S. Sanchez-Cortes and J. V. Garcia-Ramos, J. Mol. Struct., 2009, 918, 129–137 CrossRef CAS.
- L. G. Crane, D. X. Wang, L. M. Sears, B. Heyns and K. Carron, Anal. Chem., 1995, 67, 360–364 CrossRef CAS.
- A. Burneau and B. Teiten, Vib. Spectrosc., 1999, 21, 97–109 CrossRef CAS.
- L. Szabo, K. Herman, N. E. Mircescu, A. Falamas, L. F. Leopold, N. Leopold, C. Buzumurga and V. Chis, Spectrochim. Acta, Part A, 2012, 93, 266–273 CrossRef CAS PubMed.
- K. Q. Peng, Y. J. Yan, S. P. Gao and J. Zhu, Adv. Funct. Mater., 2003, 13, 127–132 CrossRef CAS.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261–3266 CrossRef CAS PubMed.
- T. Murphy, H. Schmidt and H. D. Kronfeldt, Appl. Phys. B: Lasers Opt., 1999, 69, 147–150 CrossRef CAS.
- B. Teiten and A. Burneau, J. Raman Spectrosc., 1997, 28, 879–884 CrossRef CAS.
- J. Wang, X. Wu, C. Wang, N. Shao, P. Dong, R. Xiao and S. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 20919–20929 CAS.
- C. Song, L. Min, N. Zhou, Y. Yang, S. Su, W. Huang and L. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 21842–21850 CAS.
- T. W. Lin, H. Y. Wu, T. T. Tasi, Y. H. Lai and H. H. Shen, Phys. Chem. Chem. Phys., 2015, 17, 18443–18448 RSC.
- Y. M. Lee, K. Kim and K. S. Shin, J. Phys. Chem. C, 2008, 112, 10715–10720 CAS.
- W. H. Do, C. J. Lee, D. Y. Kim and M. J. Jung, J. Ind. Eng. Chem., 2012, 18, 2141–2146 CrossRef CAS.
- A. Calloni, A. Brambilla, G. Berti, G. Bussetti, E. V. Canesi, M. Binda, A. Petrozza, M. Finazzi, F. Ciccacci and L. Duò, Langmuir, 2013, 29, 8302–8310 CrossRef CAS PubMed.
- S. Liu, Y. Li, X. Zhao, X. Liu and M. Chen, Spectrochim. Acta, Part A, 2011, 82, 205–212 CrossRef CAS PubMed.
- X. Xue, D. Xu, W. Ruan, L. Chen, L. Chang and B. Zhao, RSC Adv., 2015, 5, 64235–64239 RSC.
- H. Hu, W. Song, W. Ruan, Y. Wang, X. Wang, W. Xu, B. Zhao and Y. Ozaki, J. Colloid Interface Sci., 2010, 344, 251–255 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra28041a |
| This journal is © The Royal Society of Chemistry 2016 |