Characterization of a versatile nitrile hydratase of the neonicotinoid thiacloprid-degrading bacterium Ensifer meliloti CGMCC 7333
Abstract
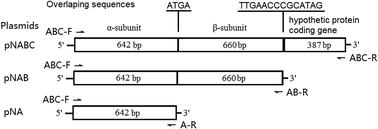
The nitrogen-fixing bacterium Ensifer meliloti CGMCC 7333 and its nitrile hydratase (NHase) degrade the neonicotinoid insecticides, thiacloprid (THI) and acetamiprid (ACE), to their corresponding amide metabolites. The NHase gene cluster is composed of α-subunit and β-subunit genes and a hypothetical protein gene. The functionality of the hypothetical protein downstream of the NHase coding genes and the characteristics of CGMCC 7333 NHase were explored in this study. Co-expression of the hypothetical protein coding gene with NHase (α- and β-subunit genes) in Escherichia coli Rosetta enhanced NHase hydration of THI and ACE two- and four-fold, respectively, and also significantly improved NHase solubility compared with the absence of the hypothetical protein coding gene. The NHase displayed an optimal reaction temperature of 50 °C for THI hydration and was unstable when the incubation temperature exceeded 40 °C. The optimum reaction pH was 7.0 and the NHase activity was stable in the pH range of 6 to 9. The enzyme activity for THI hydration was slightly inhibited by copper, zinc, and iron, and decreased by 68.6%, 75.7%, and 70.3% when 2% ethanol, ethyl acetate, and acetone were added to the reaction mixture, respectively, whereas dichloromethane and trichloromethane had no effect. The Km and kcat values of CGMCC 7333 NHase for THI hydration were 12.39 mmol L−1 and 131.36 s−1, respectively. Substrate specificity analysis indicated that CGMCC 7333 NHase also transformed 3-cyanopyridine, benzonitrile, and indole-3-acetonitrile to the corresponding amide products, with maximum specific activities of 652.52, 255.32, and 263.93 U mg−1 protein, respectively.

 Please wait while we load your content...
Please wait while we load your content...