Structure and electrochemical properties of Sm-doped Li4Ti5O12 as anode material for lithium-ion batteries
Zhanyu Lia,
Jianling Li*a,
Yuguang Zhaoa,
Kai Yangb,
Fei Gaob and
Xiao Lib
aSchool of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, No. 30 College Road, Beijing 100083, China. E-mail: lijianling@ustb.edu.cn; lizhanyu818106@163.com; 763419055@qq.com
bChina Electric Power Research Institute, Beijing 100085, China. E-mail: yangkai@epri.sgcc.com.cn; gaofei2@epri.sgcc.com.cn; m18265380882@163.com
First published on 21st January 2016
Abstract
Sm-doped Li4Ti5O12 (LTO) in the form of Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.10) is synthesized successfully by a simple solid-state reaction in air. XRD analysis and Rietveld refinement demonstrate that traces of the doped Sm3+ ions have successfully entered the lattice structure of the bulk LTO and the Sm doping does not change the spinel structure of LTO. However, of interest is that the lattice parameter increases gradually with the increase of the Sm doping amount, which is potentially beneficial for intercalation and de-intercalation of lithium ions. XPS results further identify the existence of Ti3+ ions and the transition of a small quantity of Ti ions from Ti4+ to Ti3+, which will improve the conductivity of LTO. All materials are well crystallized with a uniform and narrow size distribution in the range of 0.5–1.2 μm. The results of electrochemical measurement reveal that the Sm doping can improve the rate capability and cycling stability of LTO. Among all samples, Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) exhibits the best electrochemical properties. The specific capacities of the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) sample at charge and discharge rates of 5C and 10C are 131.1 mA h g−1 and 119.2 mA h g−1, respectively, compared with 64 mA h g−1 (5C) and 47 mA h g−1 (10C) for the pristine LTO in the potential range 1.0–2.5 V (vs. Li/Li+). This result can be attributed to Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) with a diffusion coefficient of 1.3 × 10−12 cm2 s−1, which is higher than the 7.4 × 10−14 cm2 s−1 for the LTO electrode without Sm doping. In the meantime, the discharge capacity of Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) can still reach 125.1 mA h g−1 even after 100 cycles and maintain 95.2% of its initial discharge capacity at 5C. Therefore, Sm doping has a great impact on discharge capacity, rate capability and cycling performance of LTO anode materials for lithium-ion batteries.
1. Introduction
Along with the continuous reduction of traditional energy and its harm to the environment, human beings are facing a serious challenge to realize the sustainable development of economy and society. As a result, lithium-ion batteries (LIBs) as a kind of clean energy are receiving more and more attention across the whole world.1–3 The overall requirements of a lithium-ion battery are long life, low cost, high security, high specific energy, wide temperature range, and high efficiency, of which the most important requirements are the long life, low cost, and high security.4,5 Currently, carbonaceous materials have been used as the most common commercial anode material for LIBs. However, carbonaceous materials as anode material have a lot of problems in their safety and life. Therefore, alternative anode materials with higher safety and excellent rate capability have been intensively investigated.6,7Compared with carbonaceous materials, spinel-type Li4Ti5O12 (LTO) materials show greater advantages in terms of being long life, low cost, and high security. First, they have a high insertion potential at around 1.55 V (vs. Li/Li+), which can avoid the formation of SEI layers and electroplating of lithium.8 Second, they are “zero-strain” materials, which can result in negligible changes in the unit cell volume during lithium-ion intercalation and de-intercalation.9 Last but not least, Ti is an abundant element, allowing them to be cost-effective materials.10 Unfortunately, some kinetic problems with low electrical conductivity (ca. 10−13 S cm−1) and lithium diffusion coefficient (ca. 10−9 to 10−16 cm2 s−1) limit their full capacity at high charge–discharge rates.11
To date, tremendous efforts have been made to circumvent the drawbacks of LTO. One effective strategy is reducing the particle size of LTO or preparing nanostructured LTO with various morphologies (such as a hollow or porous structure).12,13 Another effective strategy is doping with metal ions (such as Na+ and K+,14 Mg2+,15 Al3+,16 Mo6+,17 La3+,18 and Br−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) to enhance the electronic conductivity. However, very few studies have focused on doping with rare earth elements (such as Sm3+) to enhance the electrochemical performance of LTO in addition to Gd20 and Ce.21
19) to enhance the electronic conductivity. However, very few studies have focused on doping with rare earth elements (such as Sm3+) to enhance the electrochemical performance of LTO in addition to Gd20 and Ce.21
Herein, in this paper, Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.10) compounds were synthesized by a conventional solid-state method to improve the rate capability of the LTO electrodes. Table 1 presents the high rate charge and discharge performance of the element-doped LTO under the same conditions of the preparation method (solid-state reaction) and the voltage window (1.0–2.5 V). It is seen that the discharge capacity of our prepared Sm-doped LTO at 5C and 10C is amongst the largest capacity values ever reported for other element-doped LTO. Therefore, we believe that this material has a great practical application value in energy storage and lithium-ion batteries.
2. Experimental
2.1. Sample preparation
Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.10) compounds were synthesized by a conventional solid-state method. Li2CO3, TiO2 and Sm2O3 were used as the sources of Li, Ti and Sm, respectively. In a typical synthesis, the raw materials were mixed by ball milling in ethanol for 20 h, and then dried at 80 °C for 12 h in air. 5% excess Li was added to compensate for the Li evaporation at high temperature during synthesis. After that, the mixture was transferred into a muffle furnace under air atmosphere at 850 °C for 12 h with a slow heating rate of 2 °C min−1.2.2. Material characterization
The crystalline structures of the as-prepared powders were analyzed by power X-ray diffraction (XRD, Rigaku RINT2400 with Cu Kα radiation, λ = 0.154056 nm, Japan) at a scan rate of 2° min−1. The morphology and microstructure of the prepared samples were characterized by field-emission scanning electron microscopy (FESEM, Zeiss SuprATM 55 micro-scope, Germany) equipped with energy dispersive X-ray (EDX) spectroscopy. The chemical composition and valence variation of the prepared powder were investigated by X-ray photoelectron spectroscopy (XPS) measurements performed with a Kratos AXIS UltraDLD X-ray photoelectron spectrometer.2.3. Electrode preparation
The electrochemical properties of the as-prepared powders were tested by a galvanostatic charge–discharge test using CR2025-type coin cells assembled in an argon-filled glove box ([O2] < 1 ppm, [H2O] < 1 ppm). Pure lithium foil was used as the counter electrode. The working electrodes were composed of the prepared active material, conductive carbon black and binder (poly-vinyldifluoride, PVDF) in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in N-methyl-2-pyrrolidine (NMP) solution with a carnelian mortar, and then pasted on Cu foil using the doctor blade technique. After coating, the electrode was dried at 120° for 12 h in a vacuum oven. After that, the electrode with an active material loading of 1–2 mg cm−2 was cut into circular pieces with a diameter of 10 mm. The electrolyte used was a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) with a volume ratio of 1
10 in N-methyl-2-pyrrolidine (NMP) solution with a carnelian mortar, and then pasted on Cu foil using the doctor blade technique. After coating, the electrode was dried at 120° for 12 h in a vacuum oven. After that, the electrode with an active material loading of 1–2 mg cm−2 was cut into circular pieces with a diameter of 10 mm. The electrolyte used was a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 containing 1.0 M LiPF6 and a Celgard 2400 membrane as the separator.
1 containing 1.0 M LiPF6 and a Celgard 2400 membrane as the separator.
2.4. Electrochemical characterization
The galvanostatic charge–discharge tests were carried out using a Land battery test system (CT2001A Wuhan, China) between a cut off voltage of 2.5 V and 1.0 V. The rate capability of the as-prepared samples was measured at different rates of 0.2C (35 mA g−1), 0.5C (87.5 mA g−1), 1C (175 mA g−1), 2C (350 mA g−1), 5C (875 mA g−1) and 10C (1750 mA g−1) and the cycling tests were conducted at 5C. The cyclic voltammetry (CV) curves were measured on a VMP2 electrochemical workstation under a scan rate of 0.05 mV s−1 between 1.0 and 2.5 V (vs. Li/Li+). The electrochemical impedance spectroscopy (EIS) measurements were carried out at the cell’s open circuit voltage (OCV) with an amplitude of 10.0 mV over the frequency range from 100 kHz to 10 mHz.3. Results and discussion
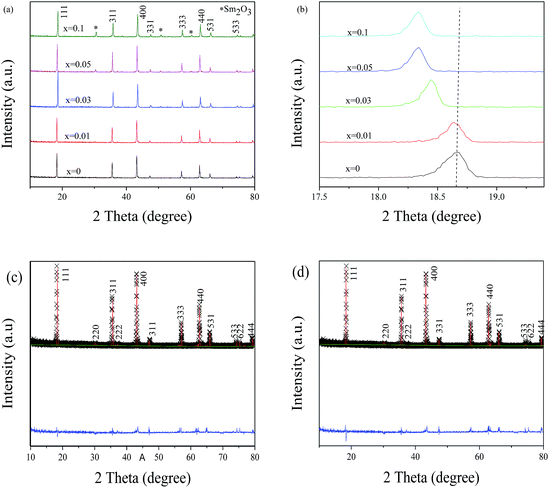
The XRD patterns of the Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.10) powders are shown in Fig. 1(a). Among all the investigated samples, the major diffraction peaks are consistent with the standard diffraction pattern of LTO (JCPDS no. 49-0207), which can be indexed to a cubic spinel structure with the space group of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, indicating that a minute amount of Sm3+ doping does not change the basic structure of LTO. No impurity peak was found for x = 0, 0.01, and 0.03, indicating that traces of the doped Sm3+ ions have successfully entered the lattice structure of the bulk LTO. However, some impurities are detectable when x = 0.1, denoting that the excess Sm cannot be doped into the LTO and then exists in the form of Sm2O3. In order to clarify this observation, the peak positions of the (111) planes of the samples are enlarged and shown in Fig. 1(b). It can be observed that a slight structural change between pristine Li4Ti5O12 and Sm-doped Li4Ti5O12 can be confirmed. Compared to pristine Li4Ti5O12, the (111) peaks of Li4−x/3Ti5−2x/3SmxO12 (x = 0.01, 0.03, 0.05 and 0.10) shift to smaller angles after Sm doping, suggesting that the lattice parameter increases gradually with the increase of the Sm doping amount. The lattice parameters were calculated from XRD, and the obtained data are listed in Table 2. In order to further confirm that Sm is indeed doped into LTO, Li4Ti5O12 and Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) Rietveld refinement patterns are shown in Fig. 1(c) and (d). Those results also show that the lattice parameter increases as x increases from 0 to 0.1. The reasons for the increase of the lattice constant after Sm modification may be explained by the following fact: a Sm3+ ion (0.0958 nm)22 has a lager ionic radius than that of Li+ (0.076 nm)23 and Ti4+ (0.0605 nm)23 in the octahedral 16d sites and then every two Ti4+ and one Li+ may be substituted by three Sm3+.20 As a result, the lattice constant is slightly enlarged, which is beneficial for intercalation and de-intercalation of lithium ions without the lattice stability being damaged. It is rational to assume that the fast Li+ diffusion caused by Sm doping will improve the electrochemical performance of the synthesized samples.
m, indicating that a minute amount of Sm3+ doping does not change the basic structure of LTO. No impurity peak was found for x = 0, 0.01, and 0.03, indicating that traces of the doped Sm3+ ions have successfully entered the lattice structure of the bulk LTO. However, some impurities are detectable when x = 0.1, denoting that the excess Sm cannot be doped into the LTO and then exists in the form of Sm2O3. In order to clarify this observation, the peak positions of the (111) planes of the samples are enlarged and shown in Fig. 1(b). It can be observed that a slight structural change between pristine Li4Ti5O12 and Sm-doped Li4Ti5O12 can be confirmed. Compared to pristine Li4Ti5O12, the (111) peaks of Li4−x/3Ti5−2x/3SmxO12 (x = 0.01, 0.03, 0.05 and 0.10) shift to smaller angles after Sm doping, suggesting that the lattice parameter increases gradually with the increase of the Sm doping amount. The lattice parameters were calculated from XRD, and the obtained data are listed in Table 2. In order to further confirm that Sm is indeed doped into LTO, Li4Ti5O12 and Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) Rietveld refinement patterns are shown in Fig. 1(c) and (d). Those results also show that the lattice parameter increases as x increases from 0 to 0.1. The reasons for the increase of the lattice constant after Sm modification may be explained by the following fact: a Sm3+ ion (0.0958 nm)22 has a lager ionic radius than that of Li+ (0.076 nm)23 and Ti4+ (0.0605 nm)23 in the octahedral 16d sites and then every two Ti4+ and one Li+ may be substituted by three Sm3+.20 As a result, the lattice constant is slightly enlarged, which is beneficial for intercalation and de-intercalation of lithium ions without the lattice stability being damaged. It is rational to assume that the fast Li+ diffusion caused by Sm doping will improve the electrochemical performance of the synthesized samples.
| Sample | x = 0 | x = 0.01 | x = 0.03 | x = 0.05 | x = 0.1 |
| Lattice parameter (Å) | 8.34715 | 8.34997 | 8.36695 | 8.37468 | 8.37965 |
In order to confirm the existence of Sm and investigate the valence variations of Ti in Sm-doped LTO (x = 0.03), the resulting powder is analyzed by XPS. The XPS spectrum of Sm (3d) in Sm-doped LTO (x = 0.03) is shown in Fig. 2(a). The two peaks at 1082.6 eV and 1111.4 eV correspond to the binding energy of Sm 3d5/2 and Sm 3d3/2, respectively, which are similar to the binding energies of Sm 3d5/2 and Sm 3d3/2 in Sm-doped LiNi0.5Mn1.5O4 (1082.9 eV and 1109.9 eV).24 The result indicates that Sm3+ does not exist in the form of Sm2O3 and has been incorporated into the Li4Ti5O12 lattice. The high resolution XPS spectrum of Ti 2p in Sm-doped LTO (x = 0.03) is shown in Fig. 2(b). There are two pairs of peaks, in which one doublet peak at 458.2 and 464.2 eV corresponds to Ti 2p3/2 and Ti 2p1/2, respectively, demonstrating the existence of Ti4+, and the other pair of peaks at 457.1 eV and 462.4 eV belongs to Ti 2p3/2 and Ti 2p1/2, respectively, indicating the existence of Ti3+ in Fig. 2. Thus, it is vigorously confirmed that there is a transition of a small quantity of Ti ions from Ti4+ to Ti3+ because of Sm doping, however, some oxygen vacancies can be generated in order to maintain the charge balance. It is well known that the existence of some oxygen vacancies or valence state changes of Ti ions from Ti4+ to Ti3+ due to the ion doping will be beneficial to the improvement of the electrochemical properties, which has been mentioned many times in the literature.25,26
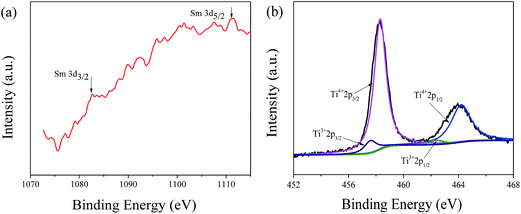 | ||
| Fig. 2 (a) High resolution XPS spectrum of Sm 3d in Sm-doped LTO (x = 0.03) and (b) high resolution XPS spectrum of Ti 2p in Sm-doped LTO (x = 0.03). | ||
In order to further investigate the structure of LTO and the influence of Sm3+ doping, a structure model has been made according to CIF file which has been reported.27 The structures of Li4Ti5O12 and Li7Ti5O12 are shown in Fig. 3. It is obvious from Fig. 3 (left) that the tetrahedral 8a sites are occupied by three quarters of the Li+ ions, the rest of the Li+ ions and Ti4+ ions occupy 1/6 and 5/6 of the octahedral 16d sites, and the oxygen atoms occupy the 32e sites, respectively. So the stoichiometry of the Li4Ti5O12 structure can be expressed as [Li]8a[Li1/3Ti5/3]16d[O4]32e (Fig. 3 (left)). Three external Li+ ions and the internal Li+ ion in the tetrahedral 8a sites are embedded into the vacant 16c sites with the discharge process, and then the stoichiometry of the Li7Ti5O12 structure can be written as [Li2]16c[Li1/3Ti5/3]16d[O4]32e (Fig. 3 (right)). As mentioned above, the existence of some oxygen vacancies and valence state changes of the Ti ions from Ti4+ to Ti3+ can be generated due to Sm modification. Therefore, it is believed that Sm3+ ions in the spinel structure of LTO will further improve the electrochemical performance.
The morphologies of the as-prepared Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.1) samples were characterized by SEM as shown in Fig. 4. It is obvious that the images of the Sm-doped and undoped LTO powders are similar, and they are all composed of irregular particles, which mainly originates from the solid-state synthesis. All materials are well crystallized with a uniform and narrow size distribution in the range of 0.5–1.2 μm. The average particle size is about 0.8 μm. The energy dispersive X-ray (EDX) spectrum (Fig. 5(a)) for Sm-doped LTO (x = 0.03) indicates the existence of O, Ti, and Nb. The EDX elemental mapping in Fig. 5(c)–(e) suggests that the distribution of the Sm element is consistent with that of Ti and O, indicating that the Sm element is uniformly distributed in Sm-doped LTO (x = 0.03).
 | ||
| Fig. 4 SEM images of the as-prepared Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.1) samples: (a) x = 0, (b) x = 0.01, (c) x = 0.03, (d) x = 0.05 and (e) x = 0.1. | ||
In order to confirm the effect of Sm doping on improving the rate capability of the as-prepared electrodes, the rate capability of the Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.1) samples at different rates of 0.2C, 0.5C, 1C, 2C, 5C and 10C are shown in Fig. 6. The charge–discharge processes of the electrodes for each stage were subject to 3 cycles. It can be obviously seen that the discharge capacity decreases as the discharge current rate increases from 0.2C to 10C. However, the undoped LTO sample capacity decreases dramatically compared to that of the Sm-doped samples with the increasing rate. The first discharge capacity of the pure LTO sample at 0.2C, 0.5C, 1C, 2C, 5C, and 10C charge–discharge rate is 162.7 mA h g−1, 142.1 mA h g−1, 118.4 mA h g−1, 91.7 mA h g−1, 64 mA h g−1 and 47 mA h g−1, respectively, while the Sm-doped samples display relatively higher capacity at the rates ranging from 0.2 to 10C. Among the Sm-doped samples, Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) shows the best high rate capability. At the 0.1C rate, its initial discharge capacity is 173.1 mA h g−1, which is closer to the theoretical capacity (175 mA h g−1) than that of the undoped LTO sample. At 5C, the de-lithiation capacity is 131.1 mA h g−1, which is 75.7% of the initial discharge capacity at 0.2C. Meanwhile pure LTO shows a much lower capacity, which is only 39.3% of that at 0.2C. Even at 10C, the discharge capacity of the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) sample is 119.2 mA h g−1 (about 68.9% retention of the initial discharge capacity at 0.2C), which is higher than that of pristine LTO. However, as the current rate returned to 0.2C again, a stable capacity of 173.1 mA h g−1 can be obtained without any decay, demonstrating that the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) sample has a good reversibility.
 | ||
| Fig. 6 Rate capabilities of the pure LTO and Li4−x/3Ti5−2x/3SmxO12 (x = 0.01, 0.03, 0.05 and 0.1) samples at different cycling rates. | ||
The initial charge–discharge curves of the pure LTO and Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) powders at different rates from 0.2 to 10C in a potential window between 2.5 and 1.0 V are shown in Fig. 7. It is clearly observed that the discharge plateau decreases and the charge platform increases with increasing the charge–discharge current density for both the electrodes, indicating that the overpotential is increasing between the electrode and the electrolyte. The charge–discharge curves of the doped and undoped LTO electrodes display distinct potential plateaus around 1.5 V (vs. Li/Li+) at 0.2C, 0.5C, 1C and 2C, corresponding to the two-phase insertion reaction between Li4Ti5O12 and Li7Ti5O12.28 However, the discharge plateau becomes inconspicuously slow with increasing the current density and even no obvious discharge plateaus can be found for pure LTO at 10C. The main reason is perhaps due to the high resistance of the electrode, which causes the high polarization of the electrode.29 Compared with the pure LTO, the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) powders show a relatively obvious discharge plateau at 10C, demonstrating that the electrochemical polarization of LTO is decreased by Sm doping.
 | ||
| Fig. 7 The initial charge–discharge curves of the pure LTO and Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) powders at different rates. | ||
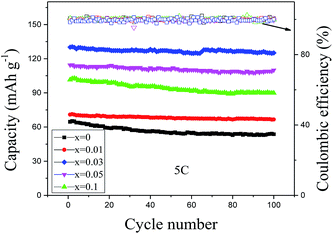
The cycling performances of the pure Li4Ti5O12 and Li4−x/3Ti5−2x/3SmxO12 (x = 0.01, 0.03, 0.05 and 0.1) at 5C are shown in Fig. 8. It is clearly observed that the Sm-doped LTO electrodes exhibit higher reversible capacities than that of pristine LTO and the coulombic efficiency in the 100 charge–discharge cycles remains almost constant at about 100%. The reason is that the lattice constant increases due to the doping of Sm, which increases the discharge capacity of LTO due to it being beneficial to the insertion and extraction of Li+ ions. Nevertheless, Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) shows the best high rate capability among the Sm-doped samples. It may be due to the fact that an increasing amount of Sm2O3 secondary phase is present in the Li4−x/3Ti5−2x/3SmxO12 (x = 0.05 and 0.1) samples. It is also obvious that the discharge capacity of Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) at 5C can still reach 125.1 mA h g−1 even after 100 cycles and maintain 95.2% of its initial discharge capacity, while it decreased to 53.6 mA h g−1 with a capacity retention of only 83% for the pure LTO. It is well known that almost all the electrochemical energy comes from the reversible redox reactions between Ti3+ and Ti4+.30 Therefore, the increase of the doped Sm can decrease the amount of active titanium ions, and then reduce the discharge capacity. Hence, according to the results obtained above, the Sm-doped LTO is considered to be an excellent electrode material.
In order to confirm that the redox reaction of LTO was not affected by Sm doping, the cyclic voltammograms of all examples as active materials at the scanning rate of 0.05 mV s−1 between 1.0 and 2.5 V are shown in Fig. 9. There is only a pair of sharp and reversible redox peaks in all the curves, indicating the good electrode kinetics of all anodes. The differences among these cyclic voltammograms are very small, suggesting that the electrochemical reaction process of LTO was not changed by Sm doping. Among all samples, the cathodic peak corresponding to Li+ intercalated into the LTO anode is located at ∼1.52 V (vs. Li/Li+) and the anodic peak corresponding to the Li+ de-intercalated from the anode is located at ∼1.65 V (vs. Li/Li+).31 The potential differences between the anodic and cathodic peaks for the as-prepared electrodes are given in Table 3. It can be seen that the potential differences between the anodic and cathodic peaks for the Sm-doped LTO samples are lower than those of LTO, revealing that Sm doping is beneficial for the reversible intercalation and de-intercalation of Li+, which is in accordance with the results described above. It is clearly observed that the oxidation and reduction peaks of Li4−1/3xTi5−2/3xSmxO12 (x = 0.03) are located at 1.635 V and 1.513 V, respectively, and the polarization is 122 mV, which is the smallest in all the Sm-doped LTO examples. However, pristine LTO has a broadened peak, suggesting that the reversibility of LTO is improved due to Sm doping. As a result, it can be expected that Sm-doped LTO electrodes possess more excellent kinetics and faster Li-ion intercalation and de-intercalation than that of pure LTO.
 | ||
| Fig. 9 CV curves of Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.1). Scan rate: 0.05 mV s−1. | ||
| Sample | φa (V) | φc (V) | φa − φc (mV) |
|---|---|---|---|
| x = 0 | 1.654 | 1.503 | 151 |
| x = 0.01 | 1.642 | 1.514 | 128 |
| x = 0.03 | 1.635 | 1.513 | 122 |
| x = 0.05 | 1.649 | 1.513 | 136 |
| x = 0.1 | 1.649 | 1.505 | 144 |
To further confirm the effect of Sm doping on the transport kinetics of lithium ions, electrochemical impedance spectroscopy (EIS) measurements for the Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.1) electrodes are conducted, and the corresponding Nyquist plots are displayed in Fig. 10(a). All the Nyquist plots exhibit one semicircle at the high to intermediate frequency range and a sloping straight line in the lowest frequency range. The diameter of the semicircle is mainly related to the charge transfer resistance (Rct) at the active material interface, associated with the interfacial electrochemical reaction activity, and the sloping straight line in the low frequency region corresponds to the Warburg impedance caused by semi-infinite diffusion of Li+ ions in the electrode.32 The experimental results can be fitted using the equivalent circuit (inset of Fig. 10(a)). In the equivalent circuit, Rs is the ohmic resistance from the electrolyte, Rct is the charge transfer resistance related to lithium-ion interfacial transfer, CPE is the constant phase element related to the interfacial capacitance and W is the Warburg impedance of solid-phase diffusion associated with Li-ion diffusion kinetics,33 and the fitted Rct and Rs data are listed in Table 4. It can be found that Rct of the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) electrode is lowest in all the examples, suggesting that an appropriate amount of Sm dopant is favorable to improve the electronic conductivity of the LTO.
| Sample | Rs (Ω) | Rct (Ω) | DLi (cm2 s−1) |
|---|---|---|---|
| x = 0 | 2.147 | 94.1 | 7.4 × 10−14 |
| x = 0.01 | 1.78 | 68.26 | 1.8 × 10−13 |
| x = 0.03 | 1.34 | 43.58 | 1.3 × 10−12 |
| x = 0.05 | 2.13 | 53.98 | 1.1 × 10−12 |
| x = 0.1 | 2.45 | 58.41 | 3.6 × 10−13 |
From Fig. 10(a), it can be found that the sizes of the semicircles for Sm-doped LTO are smaller than that of pure LTO, suggesting that Sm-doped LTO possess smaller charge transfer resistance compared with pure LTO, which could be attributed to enhanced electrical conductivity due to Sm doping. The lithium ion diffusion coefficient (DLi) can be calculated from the plots in the low frequency region according to the following equations:32,34,35
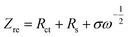 | (1) |
 | (2) |
4. Conclusions
In this paper, we have successfully synthesized well-structured cubic spinel Li4−x/3Ti5−2x/3SmxO12 (x = 0, 0.01, 0.03, 0.05 and 0.1) samples by a conventional solid-state reaction in air atmosphere. Sm doping did not change the spinel structure of LTO. However, only some of the Sm can enter the lattice structure of LTO and an excessive amount of Sm exists in the form of Sm2O3. Among the Sm-doped samples, Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) exhibits superior high rate capability and excellent cyclability. Even at 10C, the discharge capacity of the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) sample is 119.2 mA h g−1 (about 68.9% retention of the initial discharge capacity at 0.2C), which is higher than that of pristine LTO. Compared with the pure LTO, the Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) powders show a smaller electrochemical polarization at a high rate charge–discharge process demonstrating that the resistance of the electrode is decreased by Sm doping. Therefore, the outstanding electrochemical properties of Li4−x/3Ti5−2x/3SmxO12 (x = 0.03) make it a promising anode material for power lithium-ion batteries.Acknowledgements
The work was financially supported by the science and technology project of State Grid Corporation of china (DG71-15-042).References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- Y. Xia, Z. Xiao, X. Dou, H. Huang, X. H. Lu, R. J. Yan, Y. P. Gan, W. J. Zhu, J. P. Tu, W. K. Zhang and X. Y. Tao, ACS Nano, 2013, 7, 7083–7092 CrossRef CAS PubMed.
- G. H. Jeong, H. B. Bae, D. Choi, Y. H. Kim, S. Yoon and S. W. Kim, J. Phys. Chem. C, 2012, 116, 23851–23857 CAS.
- A. S. Prakash, P. Manikandan, K. Ramesha, M. Sathiya, J. M. Tarascon and A. K. Shukla, Chem. Mater., 2012, 22, 2857–2863 CrossRef.
- K. Amine, I. Belharouak, Z. Chen, T. Tran, H. Yumoto, N. Ota, S. T. Myung and Y. K. Sun, Adv. Mater., 2010, 22, 3052–3057 CrossRef CAS PubMed.
- W. J. Zhu, H. Huang, Y. P. Gan, X. Y. Tao, Y. Xia and W. K. Zhang, Electrochim. Acta, 2014, 138, 376–382 CrossRef CAS.
- T. F. Yi, S. Y. Yang and Y. Xie, J. Mater. Chem. A, 2015, 3, 5750–5777 CAS.
- Z. Y. Jia, Q. Zhou, X. W. Li, Y. Fu, H. Ming and J. W. Zheng, Electrochim. Acta, 2015, 156, 216–222 CrossRef CAS.
- J. Q. Wang, Z. Z. Yang, W. H. Li, X. W. Zhong, L. Gu and Y. Yu, J. Power Sources, 2014, 266, 323–331 CrossRef CAS.
- T. Ohzuku, A. Ueda and N. Yamamoto, J. Electrochem. Soc., 1995, 142, 1431–1435 CrossRef CAS.
- C. Y. Ouyang, Z. Y. Zhong and M. S. Lei, Electrochem. Commun., 2007, 9, 1107–1112 CrossRef CAS.
- L. Y. Cao, Y. N. Hui, H. B. Ouyang, J. F. Huang, Z. W. Xu, J. Y. Li, W. Z. Zhang, S. M. Chai and S. W. Guo, RSC Adv., 2015, 5, 35643–35650 RSC.
- L. Yu, H. B. Wu and X. W. (David) Lou, Adv. Mater., 2014, 25, 2296–2300 CrossRef PubMed.
- Z. X. Liu, L. M. Sun, W. Y. Yang, J. B. Yang, S. B. Han, D. F. Chen, Y. T. Liu and X. F. Liu, Solid State Sci., 2015, 44, 39–44 CrossRef CAS.
- S. Z. Ji, J. Y. Zhang, W. W. Wang, Y. Huang, Z. R. Feng, Z. T. Zhang and Z. L. Tang, Mater. Chem. Phys., 2010, 123, 510–515 CrossRef CAS.
- D. Li, Y. Sun, X. Z. Liu, R. W. Peng and H. S. Zhou, Chem. Sci., 2015, 6, 4066–4070 RSC.
- T. F. Yi, Y. Xie, L. J. Jiang, J. Shu, C. B. Yue, A. N. Zhou and M. F. Ye, RSC Adv., 2012, 2, 3541–3547 RSC.
- S. Y. Yang, J. Yuan, Y. R. Zhu, T. F. Yi and Y. Xie, Ceram. Int., 2015, 41, 7073–7079 CrossRef CAS.
- J. Q. Wang, Z. Z. Yang, W. H. Li, X. W. Zhong, L. Gu and Y. Yu, J. Power Sources, 2014, 266, 323–331 CrossRef CAS.
- Q. Y. Zhang, M. G. Verde, J. K. Seo, X. Li and Y. S. Meng, J. Power Sources, 2015, 280, 355–362 CrossRef CAS.
- T. P. Zhou, X. Y. Feng, X. Guo, W. W. Wu, S. Cheng and H. F. Xiang, Electrochim. Acta, 2015, 174, 369–374 CrossRef CAS.
- H. B. Sun, Y. G. Chen, C. H. Xu, D. Zhu and L. H. Huang, J. Solid State Electrochem., 2012, 16, 1247–1254 CrossRef CAS.
- T. F. Yi, H. P. Liu, Y. R. Zhu, L. J. Jiang, Y. Xie and R. S. Zhu, J. Power Sources, 2012, 215, 258–265 CrossRef CAS.
- M. Y. Mo, K. S. Hui, X. T. Hong, J. S. Guo, C. C. Ye, A. J. Li, N. Q. Hu, Z. Z. Huang, J. H. Jiang, J. Z. Liang and H. Y. Chen, Appl. Surf. Sci., 2014, 290, 412–418 CrossRef CAS.
- Y. L. Qi, Y. D. Huang, D. Z. Jia, S. J. Bao and Z. P. Guo, Electrochim. Acta, 2009, 54, 4772–4776 CrossRef CAS.
- J. Wolfenstine and J. L. Allen, J. Power Sources, 2008, 180, 582–585 CrossRef CAS.
- A. Deschanvres, B. Raveau and Z. Sekkal, Mater. Res. Bull., 1971, 6, 699–704 CrossRef CAS.
- D. Pal and J. L. Pandey, Bull. Mater. Sci., 2010, 33, 691–696 CrossRef CAS.
- D. Wang, C. M. Zhang, Y. Y. Zhang, J. Wang and D. N. He, Ceram. Int., 2013, 39, 5145–5149 CrossRef CAS.
- Q. Y. Zhang, C. L. Zhang, B. Li, D. D. Jiang, S. F. Kang, X. Li and Y. G. Wang, Electrochim. Acta, 2013, 107, 139–146 CrossRef CAS.
- Y. Y. Zhang, C. M. Zhang, Y. Lin, D. B. Xiong, D. Wang, X. Y. Wu and D. N. He, J. Power Sources, 2014, 250, 50–57 CrossRef CAS.
- W. J. Zhu, H. Yang, W. K. Zhang, H. Huang, X. Y. Tao, Y. Xia, Y. P. Gan and X. Z. Guo, RSC Adv., 2015, 5, 74774–74782 RSC.
- A. Y. Shenouda and K. R. Murali, J. Power Sources, 2012, 214, 220–226 CrossRef.
- S. L. Chou, J. Z. Wang, H. K. Li and S. X. Dou, J. Phys. Chem. C, 2011, 115, 16220–16227 CAS.
- J. Chen, L. Yang, S. Fang, S. i. Hirano and K. Tachibana, J. Power Sources, 2012, 200, 59–66 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |