Jacalin-capped silver nanoparticles minimize the dosage use of the anticancer drug, shikonin derivatives, against human chronic myeloid leukemia†
Abstract
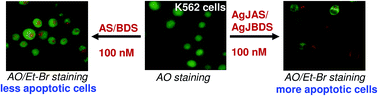
Repeated consumption of a chemotherapeutic drug in high doses often leads to drug resistance. The objective of this study was to develop a facile method to enhance the anticancer activity of the phytomolecules, acetylshikonin (AS) and beta,beta-dimethylacrylshikonin (BDS). Herein, we demonstrated that jacalin-capped silver nanoparticles (JAgNPs) loaded with AS/BDS induce maximum cytotoxicity effects on human chronic myeloid leukemia (CML), K562 at low concentration (100 nM), whereas for a similar effect about 500 nM of pure AS/BDS was required. Fluorescence microscopy data revealed the internalization of JAgNPs-AS/BDS complex into K562 cells. The intracellular localization of the drug caused the production of excess reactive oxygen species (ROS), elevation in the secretion of tumor necrosis factor (TNF-α), suppression in the production of interleukin-10 (IL-10), losses of mitochondrial membrane potential, DNA damage and apoptosis. The effective role of ROS and TNF-α in JAgNPs-AS/BDS mediated cell death was proven by pretreatment of cells with N-acetyl cysteine, a ROS scavenger, and pentoxifylline, a potent TNF-α blocker. The mode of K562 cell death was confirmed by annexin-FITC/PI staining followed by flow-cytometric analysis. Further, we disclosed the contribution of different caspase activation pathways in TNF-α mediated cell death. Taken together, our study elucidated that the judicious use of AgNPs might improve the therapeutic efficacy of AS/BDS against CML at lower doses.


 Please wait while we load your content...
Please wait while we load your content...