Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave-enhanced aqueous biphasic dehydration of carbohydrates to 5-hydroxymethylfurfural†
P.
Wrigstedt
,
J.
Keskiväli
and
T.
Repo
*
Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, P.O. Box 55, A. I. Virtasen aukio 1, FI-00014, Finland. E-mail: timo.repo@helsinki.fi
First published on 4th February 2016
Abstract
We describe herein an efficient microwave-assisted aqueous biphasic dehydration of carbohydrates to 5-hydroxymethylfurfural (HMF). The effects of several alkali metal salts in aqueous phase, organic solvents as an extractive phase and Lewis acids are evaluated on the reaction. Specifically, starting from fructose, the use of bromide salts in aqueous phase and the common organic solvent MeCN or lignocellulose-derived γ-valerolactone (GVL) as organic extractors are highly beneficial, leading to excellent HMF yields of up to 91% with HCl as a Brønsted acid catalyst. In conjunction with an isomerization catalyst, the method was applicable to glucose as well as various disaccharides and cellulose, affording HMF in notably good yields, particularly with GVL as an extractor and reusable Amberlyst-38(wet) as an acid catalyst. The exceptionally high HMF yields obtained in aqueous solutions is attributed to the combined effect of the biphasic reaction system and the application of microwaves, which ensures short reaction times and minimized by-product formation thereof.
Introduction
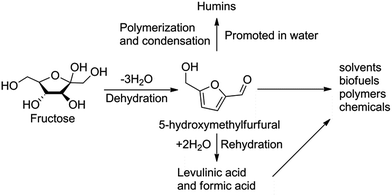
Over the last decade the dehydration of carbohydrates to 5-hydroxymethylfurfural (HMF) has attracted increasing attention for the sustainable production of chemicals (Scheme 1).1 For instance, HMF can be oxidized to 2,5-furandicarboxylic acid as a potential replacement for terephthalic acid in the production of polyesters,2 or converted to high energy density biofuel dimethylfuran.3Dehydration of fructose to HMF using both homogeneous and heterogeneous catalytic systems has been investigated extensively.4 Moderate to excellent yields of HMF have been achieved using mineral acids,5 ion exchange resins,6 oxides7 and zeolites8 as catalysts in monophasic ionic liquids,9 high-boiling organic solvents (e.g. dimethylacetamide5 and DMSO10) and their mixtures.11 However, the high cost of the solvents, and high downstream separation costs limits their economic feasibility. In this respect, aqueous processes are favored yet unfortunately inefficient (HMF yields < 50%) because side reactions, such as polymer formation and HMF rehydration into levulinic acid and formic acid, are promoted in water (Scheme 1).12 Consequently, the challenge is to make aqueous processes more efficient, that is, to increase the HMF selectivity by reducing the formation of by-products during the reaction. The development of various water–organic biphasic reaction systems, such as water–MIBK,13 and water–(MIBK + 2-BuOH)14 systems, resulted in fair HMF yields of 69% and 60%, respectively. Systems with additional modifiers in aqueous phase, such as DMSO,15 NMP16 or PVP15 resulted in very good HMF yields of up to 83%. However, contamination of the organic phase by the modifier can lead to complex separation of solvent and product at the end of the process.
Recently, the presence of alkali metal salts, such as NaCl and KBr, has been reported to improve the HMF yields in glucose dehydration in organic solvents5 and aqueous biphasic systems.17 This was ascribed to the Cl− and Br− anions influencing the reaction by enhancing the fructose dehydration step (the reaction proceeds through fructose intermediate).5 Also, in aqueous biphasic systems the addition of an excess of salt is beneficial in increasing the HMF distribution to organic phase during the reaction (salting-out effect), and enabling the use of water-miscible solvents as the organic phase, such as THF and MeCN, which are expected to dissolve HMF better than common water-immiscible solvents. Exploiting this strategy with fructose, Hansen and co-workers reported an increase in HMF yield from 28% to 45% by the addition of NaCl into the H3BO3(aq)–MIBK biphasic system.18 Cao et al. described the use of NaHSO4(aq)–THF biphasic solvent system with tetraethyl ammonium salts, obtaining HMF yield of 81%.19 Recently, high HMF yield of 88% was reported for (IL-HSO4)(aq)–MeCN system with acidic ionic liquid (IL) as excess salt.20
In general, aqueous acid-catalyzed dehydration reaction is fairly slow process and high temperature is commonly required for high product yield. In contrast to conventional heating, microwave irradiation generates high input of energy, thus rapidly overcoming the energy barrier necessary for product formation. In many occasions, microwave processing has been shown to dramatically reduce reaction times and to increase product yields and purities compared to conventionally heated experiments.21 In this respect, the implementation of microwaves appears to be a method of choice for accelerating the fructose dehydration reaction. To this date, only few reports4 describe the microwave-assisted synthesis of HMF from fructose or glucose employing aqueous mono22 or biphasic22c,23 conditions.
Herein, we report high-yield transformation of carbohydrates to HMF applying the combination of microwaves and biphasic reaction systems.
Results and discussion
The role of alkali metal salts in the fructose dehydration to HMF is well-established in organic solvents, in which bromide anions (NaBr or KBr) in place of chlorides (NaCl or KCl) enhance the reaction resulting in higher HMF yields.5,24 This was ascribed to bromide anions promoting 1,2-enol formation from fructofuranosyl oxocarbenium ion, generated by Brønsted acid-catalyzed dehydration of C2 in fructose, more efficiently than the corresponding chlorides.5 However, in aqueous mono or biphasic media the results of the impact of salts are not consistent.4,17 For example, chlorides performed better in the fructose dehydration to HMF using (boric acid + salt)(aq)–MIBK18 or HCl(aq)–nBuOH biphasic system,25 whereas better HMF yields were reported with bromides in (CrCl3·6H2O + salt)(aq)–(acetone + toluene) system.17 Therefore, we initiated the studies by investigating the effect of different sodium and potassium salts in an aqueous phase and microwave-compatible organic solvents as an extractive phase on the fructose conversion to HMF at 160 °C in the presence of 0.1 M HCl catalyst (Table 1).| Entry | Salt b | Organic phase | Conversion (%) | Yield (%) |
|---|---|---|---|---|
a Reaction conditions: ∼10 wt% fructose in (0.1 M HCl–salt)(aq)–solvent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v, MW 160 °C, 1 min.
b Saturated solution.
c Without HCl.
d GVL = γ-valerolactone.
e Fructose and i-PrOH peak overlapped (HPLC).
f With 5 mol% CrCl3·6H2O. 2 v/v, MW 160 °C, 1 min.
b Saturated solution.
c Without HCl.
d GVL = γ-valerolactone.
e Fructose and i-PrOH peak overlapped (HPLC).
f With 5 mol% CrCl3·6H2O.
|
||||
| 1c | KBr | MeCN | 1 | <1 |
| 2 | NaBr | MeCN | >99 | 82 |
| 3 | KBr | MeCN | >99 | 85 |
| 4 | NaCl | MeCN | 98 | 77 |
| 5 | KCl | MeCN | 97 | 78 |
| 6 | NaI | MeCN | 94 | 68 |
| 7 | KI | MeCN | 96 | 67 |
| 8 | KF | MeCN | 99 | — |
| 9 | KBr | GVLd | >99 | 84 |
| 10 | NaCl | GVL | 96 | 79 |
| 11 | KBr | THF | 95 | 76 |
| 12 | NaCl | THF | 83 | 68 |
| 13 | KBr | DMF | 11 | 6 |
| 14 | KBr | i-PrOH | n.de | 73 |
| 15 | KBr | 2-BuOH | 79 | 61 |
| 16 | KBr | MIBK/2-BuOH | 98 | 74 |
| 17f | KBr | MeCN | 96 | 79 |
In comparison to NaCl and KCl, the reactions with NaBr and KBr clearly resulted in faster fructose conversion and 6–8% higher HMF yields, regardless of the extractive solvent. The use of NaI and KI reduced the HMF yields further and no reaction occurred with the corresponding fluorides. The positive effect of halide ions on HMF yields decreased in the order Br > Cl > I ≠ F, which is in agreement with previous results using organic solvents or comparable biphasic system under conventional heating.5,17 Notably, the use of MeCN or biomass-derived γ-valerolactone (GVL), the latter of which has been extensively studied as a renewable solvent for biomass conversion,26 in place of traditionally used MIBK/2-BuOH, THF, DMF or alcoholic solvents, such as 2-BuOH and i-PrOH, as an organic phase resulted in faster fructose conversion rate and better HMF selectivity and yields. The high HMF yield of 85% and 84% obtained with KBr/MeCN and KBr/GVL systems are comparable to those of obtained in ionic liquids and high-boiling organic solvents,4 and considerably better than obtained in aqueous monophasic solutions.22a,22b Importantly, the use of those solvents as extractors prevented the formation of insoluble humins and the most common by-products levulinic acid and formic acid due to HMF rehydration (HPLC), ascribed to short reaction time and mild conditions. Also, we did not observe the presence of acetamide or acetic acid (HPLC) caused by an acid-catalyzed acetonitrile hydrolysis. It is noteworthy that after the reaction the aqueous and organic phases were fully separated, consequently allowing an easy separation of HMF. For example, the low boiling point of MeCN could be advantageous in the distillation process described by Dumesic et al. with 2-BuOH–MiBK as an extraction solvent.15 The addition of aldose-to-ketose isomerization catalyst (CrCl3·6H2O) decreased HMF yields from 85% to 79% (entry 15). Hence, the use of isomerization catalysts, such as AlIII, CrIII and LaIII halides, in the fructose dehydration is not beneficial because the metal cations converts fructose to glucose or mannose (equilibrium), which are significantly more difficult to dehydrate to HMF.4a
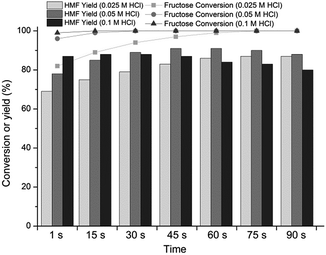
We next explored the impact of HCl concentration (0.025 M, 0.05 M and 0.1 M) on the reaction outcome using (HCl + KBr)(aq)–MeCN system at 160 °C, shown in Fig. 1. It should be noted that with MW instrument (Biotage Initiator) the heating from r.t. to 160 °C took 80 s, after which the reaction time was measured. For example, the total heating time for 1 and 60 s reactions were therefore 81 and 140 s, respectively (see Fig. S1 and S2 in ESI† for MW heating profiles).
The initial fructose conversion rate was surprisingly fast, accelerating with increasing acid concentration, and high 82%, 96% and 98% conversions were recorded in only 1 second reaction time at 160 °C. Full fructose conversions were reached with 0.025 M HCl in 75 seconds, with 0.05 M HCl in 30 seconds and with 0.1 M HCl in 15 seconds. The maximum HMF yields were linked to HCl concentration, exhibiting a tradeoff between accelerating the formation rate of HMF and increasing formation rate of the by-products (Fig. 1). Albeit the formation rate of HMF was accelerated with 0.1 M HCl in respect to 0.05 M HCl, the concurrent increase in the by-product formation rate led to lower HMF yields. Correspondingly, the deceleration in the HMF formation rate with 0.025 M HCl increased the reaction time and thus, in comparison to the reaction in 0.05 M HCl, exposed the formed HMF to by-product formation resulting in lower yields. The decomposition rate of HMF was markedly fast with 0.1 M HCl, HMF yield decreasing from 88% to 83% in 60 second time interval (see Table S2 in ESI†). After 300 seconds the HMF yield decreased to 76%, demonstrating that the acid-promoted undesired products formation was indeed very rapid under MW conditions. In the case of 0.05 M HCl the presence of intermediate dehydration products was evident as the maximum HMF yield was recorded approximately 30 seconds after the full conversion of fructose. However, we were not able to identify these intermediates by HPLC analysis.
By conducting the reaction in a single-phase manner, using catalytic amount of KBr in place of saturated solution in a 0.1 M HCl–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) system for 1 min at 160 °C, afforded HMF in surprisingly good 82% yield (see Table S1 in ESI†). Without KBr only 30% HMF yield was recorded, showing that the presence of bromide anions was crucial for good product yields. It is worth adding that the short reaction time and high yield experienced in the single phasic dehydration of fructose to HMF makes the process applicable for continuous flow manufacturing where short residence times are essential to achieve a high throughput.27 Particularly, industrial continuous flow microwave process, recently described by Morschhäuser et al., which operates under high-temperature/high-pressure conditions with throughput of 20 L h−1, could be ideal for this type of reaction.28 Obviously, the throughput of the system can be readily increased by parallel reactors.
4 v/v) system for 1 min at 160 °C, afforded HMF in surprisingly good 82% yield (see Table S1 in ESI†). Without KBr only 30% HMF yield was recorded, showing that the presence of bromide anions was crucial for good product yields. It is worth adding that the short reaction time and high yield experienced in the single phasic dehydration of fructose to HMF makes the process applicable for continuous flow manufacturing where short residence times are essential to achieve a high throughput.27 Particularly, industrial continuous flow microwave process, recently described by Morschhäuser et al., which operates under high-temperature/high-pressure conditions with throughput of 20 L h−1, could be ideal for this type of reaction.28 Obviously, the throughput of the system can be readily increased by parallel reactors.
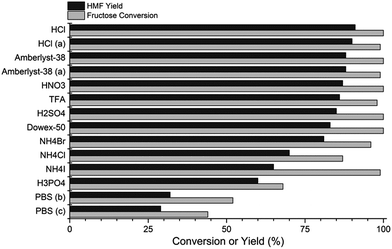
In view of the ample reports describing the reaction with different acids,4 we further attempted to improve the HMF yields by assessing the effect of different homogenous (H2SO4, HCl, HNO3, H3PO4, TFA and ammonium salts) and heterogeneous (Dowex-50 and Amberlyst-38) acid catalysts on the reaction. The experiments were performed in (acid + KBr)(aq)–MeCN system at 160 °C for 1 minute reaction time (Fig. 2). The acid concentration was set to 0.05 M except with Amberlyst-38 and Dowex-50, the amounts of which were optimized in separate experiments (see Table S4 in ESI†).
From Fig. 2, HCl was the most efficient acid, affording HMF in 91% yield with >99% fructose conversion. However, almost comparable HMF yields of 85%, 87%, 86% and 88% were recorded with H2SO4, HNO3, TFA and Amberlyst-38 catalysts, respectively. The effect of the homogeneous Brønsted acids on HMF yields decreased in order HCl > HNO3 ≥ TFA ≥ H2SO4 ≫ H3PO4. To our surprise, by replacing KBr with weakly acidic ammonium halides (NH4Cl, NH4Br and NH4I, saturated solution), the reaction gave relatively high HMF yields of up to 81% (with NH4Br) and without additional strong Brønsted acids. The solid heterogeneous acids, Dowex-50WX4 and Amberlyst-38(wet), readily dehydrated fructose furnishing HMF in very good 83% and 88% yields, respectively. The application of phosphate buffer solution (PBS, pH 2.1) as a reactive phase resulted in low HMF selectivity and yields, contrary to an earlier report employing PBS/MIBK–2-BuOH biphasic system,22c wherein very high HMF yield of 88% were reported under microwave conditions.
The main advantage in the microwave-assisted synthesis is an increase in reaction rate,29 reducing exposure time of the product to decomposition and increasing space-time-yield, which is an important feature in potential industrial applications. To assess the extent of the effect of MW irradiation on the reaction, we conducted experiments under conventional oil-bath heating at 160 °C applying biphasic (HCl + KBr)(aq)–MeCN system (Fig. 3).
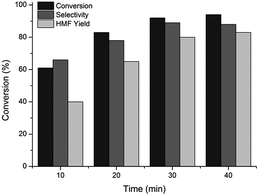 | ||
Fig. 3 Fructose dehydration to HMF under conventional oil-bath heating (conditions: ∼10 wt% fructose (0.05 M HCl + KBr)(aq)–MeCN 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v, 160 °C, 10–40 min). 2 v/v, 160 °C, 10–40 min). | ||
In comparison to MW irradiation, the conversion rate of fructose was considerably slower, agreeing with the previous report on fructose dehydration to HMF.30 After 10 min of the reaction the fructose conversion was only 62% with an HMF yield of 38%, which then gradually increased to 94% and 79% at 40 min, respectively. Likewise with MW irradiation, the presence of unidentified intermediates was obvious as the HMF selectivity increased with increasing reaction time from 64% (10 min) to 85% (30 min). Thereafter the selectivity decreased due to the governing formation rate of side-products such as humins. Similarly to the reactions conducted under MW heating, the yield of levulinic acid was negligible (<1%) in all experiments and, as a result, can be attributed to the biphasic system (MeCN). In contrast to microwave heated experiments, the presence of visible insoluble humins, the amount of which increased with time, were observed after the reaction. According to the results above, we may deduce that in comparison to conventional heating, a short-term reaction under MW irradiation is more efficient in promoting the dehydration of fructose to HMF by greatly reducing the reaction time, and consequently, the formation of by-products such as soluble and insoluble humins resulting in higher HMF selectivity and yield.
In the dehydration of fructose to HMF one of the major challenges is the acid-catalyzed by-product formation, which can be reduced by using dilute solutions.22a However, from the industrial and economical viewpoints, higher initial fructose concentrations are desirable due to the small reactor volumes, and more efficient separation and purification steps.31 Moreover, several reports have shown that microwave heating become more energy efficient as the scale increases, partly due to efficient transfer of microwave energy to a larger reaction mass.32 Therefore, we performed scale-up experiments using higher initial fructose concentrations from 10 to 50 wt%. As Fig. 4 illustrates, the fructose conversion was very high (>98%) in all experiments. In accordance with the previous reports the best HMF yield of 90% was obtained with the lowest fructose concentration.22a,22b Small decrease in HMF yield and selectivity was then observed with increasing fructose concentration gradually by 10 wt%, and the lowest HMF yield of 77% was obtained with the highest fructose concentration of 50 wt%. The HMF yield of 84% obtained with fructose concentration of 30 wt% is noticeably better than reported earlier for aqueous mono22a or biphasic systems.22c,25
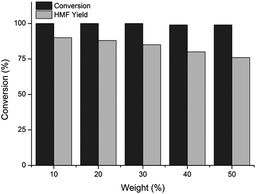 | ||
Fig. 4 Results of fructose dehydration to HMF with increasing fructose concentration (conditions: 10 to 50 wt% fructose (9 mol% HCl + KBr)(aq)–MeCN 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v, MW 160 °C, 1 min). 2 v/v, MW 160 °C, 1 min). | ||
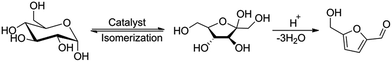
The use of glucose-to-fructose isomerization catalyst enables a one-pot tandem isomerization–dehydration process to produce HMF directly from glucose and glucose-containing carbohydrates, as depicted in Scheme 2.33 High HMF yields of up to 63% from glucose have been reported for aqueous systems involving various catalyst such as LaIII, CrIII and AlIII halides, boric acid and borates.4 In view of this, we tested the efficiency of our system, described above, in the dehydration of glucose to HMF. Accordingly, an isomerization catalysts (boric acid,18,34 sodium borate,22c CrCl3·6H2O17,35 or AlCl3·6H2O36) was added to the (acid + KBr)(aq)–GVL or (acid + KBr)(aq)–MeCN system applying Amberlyst-38(wet) or HCl as acid catalysts (Table 2).
| Entry | Time (min) | Isomerization catalyst | Glucose conversion (%) | HMF yield (%) |
|---|---|---|---|---|
a Reaction conditions: 10 mol% metal halide catalyst, 20 mol% boric acid or borate, 21 mg Amberlyst-38(wet) ∼10 wt% glucose in KBr(aq)–GVL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v, MW 160 °C, 1–4 min.
b Without Amberlyst-38(wet).
c 30 mol% AlCl3·6H2O.
d 0.05 M HCl as catalyst.
e MeCN as an organic phase.
f THF as an organic phase. 2 v/v, MW 160 °C, 1–4 min.
b Without Amberlyst-38(wet).
c 30 mol% AlCl3·6H2O.
d 0.05 M HCl as catalyst.
e MeCN as an organic phase.
f THF as an organic phase.
|
||||
| 1 | 3 | Boric acid | 30 | 10 |
| 2b | 3 | Boric acid | 1 | — |
| 3 | 3 | Sodium borate | 61 | — |
| 4 | 1 | CrCl3·6H2O | 87 | 59 |
| 5 | 2 | CrCl3·6H2O | 93 | 70 |
| 6 | 3 | CrCl3·6H2O | 98 | 74 |
| 7 | 4 | CrCl3·6H2O | 99 | 73 |
| 8 | 3 | AlCl3·6H2O | 82 | 40 |
| 9c | 3 | AlCl3·6H2O | 93 | 66 |
| 10d | 3 | CrCl3·6H2O | 98 | 71 |
| 11b | 3 | CrCl3·6H2O | 97 | 65 |
| 12e | 3 | CrCl3·6H2O | 98 | 70 |
| 13f | 3 | CrCl3·6H2O | 92 | 54 |
In contrast to fructose dehydration, we found that Amberlyst-38(wet) as an acid catalyst performed equally or slightly better than 0.05 M HCl in the reaction. Also, MeCN was found to be less suitable solvent than GVL as an organic extractive phase (constantly 1–4% better yields using GVL, See Table 2 and S5 in ESI†). The use of THF as an organic phase led to decreased HMF yield compared to those of MeCN and GVL (entry 12).
Boric acid and sodium borate (Borax) were reported as efficient catalysts in glucose-to-fructose isomerization in ionic liquid34 and biphasic aqueous phosphate buffer–(MIBK + 2-BuOH) system,22c affording HMF yields of 41% and 63%, respectively. However, when applied to our system these catalysts were inefficient. The use of boric acid gave HMF in a low 10% yield with poor selectivity after 3 minutes (entry 1). In the absence of strong Brønsted acid only 1% glucose conversion occurred (entry 2). Surprisingly, with sodium borate as catalyst no HMF was formed albeit 61% glucose conversion was observed after the reaction (entry 3). Of the isomerization catalysts investigated in this study, CrCl3·6H2O was the most efficient under MW irradiation conditions and, in agreement with previous reports, performed better than AlCl3·6H2O (entries 6, 8 and 9).17,35b Thus, the best HMF yield of 74% with glucose conversion of 98% was obtained in (CrCl3·6H2O + Amberlyst-38 + KBr)(aq)–GVL system in 3 minutes that is, up to now, the best HMF yield obtained from glucose in aqueous systems.4 As with fructose, levulinic acid was detected only in trace amounts (<1%), which is remarkably low if compared to biphasic systems with similar glucose conversions under conventional heating, wherein 5–10% of levulinic acid is typically produced.17,35a Notably, without strong Brønsted acid catalyst (0.05 M HCl or Amberlyst-38) lower HMF yield of 65% from glucose with CrCl3·6H2O was obtained (the dehydration step proceeds because of the intrinsic Brønsted acidity of CrCl3·6H2O in aqueous solutions, entry 10 vs. 11). Recently, it was reported that additional strong mineral acids affect the CrCl3·6H2O catalyzed glucose dehydration to HMF under conventional oil-bath heating at 140 °C by significantly decelerating glucose-to-fructose isomerization.17,35a This was ascribed to the restrained formation of the chromium–glucose chelate complex with increasing acidity, leading to the change in the rate-determining step from fructose dehydration to glucose-to-fructose isomerization.17 The strong retardation of the glucose-to-fructose isomerization, arising from the presence of Brønsted acid, resulted in lower substrate conversions, HMF selectivity and yield. According to our results, this tendency can be avoided by conducting the reaction rapidly at high temperature under MW irradiation.
We also studied the (Amberlyst-38 + KBr)(aq)–GVL catalytic system for the dehydration of mannose, various disaccharides (lactose, cellobiose and sucrose) and polysaccharides (inulin and cellulose) with or without CrCl3·6H2O additive, depending on the carbohydrate constituent (Table 3).
| Entry | Carbohydrate | Time (min) | HMF yield (%) |
|---|---|---|---|
a Reaction conditions: 10 mol% CrCl3·6H2O, 21 mg Amberlyst-38(wet) and 150 mg of carbohydrate in KBr(aq)–GVL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v, MW 160 °C, 1–3 min.
b MeCN as organic phase.
c 38% glucose remaining after the reaction.
d
T = 175 °C. 2 v/v, MW 160 °C, 1–3 min.
b MeCN as organic phase.
c 38% glucose remaining after the reaction.
d
T = 175 °C.
|
|||
| 1 | Mannose + CrCl3·6H2O | 3 | 69 |
| 2 | Lactose + CrCl3·6H2O | 3 | 55 |
| 3 | Cellobiose + CrCl3·6H2O | 3 | 71 |
| 4b | Cellobiose + CrCl3·6H2O | 3 | 67 |
| 5 | Sucrose + CrCl3·6H2O | 3 | 77 |
| 6c | Sucrose | 3 | 49 |
| 7 | Inulin | 1 | 80 |
| 8d | MC cellulose + CrCl3·6H2O | 3 | 40 |
HMF yields with disaccharides were only slightly lower than those of glucose under the same reaction conditions, showing that no additional strong Brønsted acid along with Amberlyst-38(wet) was necessary to promote the hydrolysis of the glycosidic linkage of disaccharides. The system worked notably well with cellobiose and inulin leading to good HMF yields of 71% and 80%, respectively. Additionally, the reaction with sucrose together with CrCl3·6H2O gave HMF in 77% yield. As expected, the absence of an isomerization catalyst reduced the HMF yield to 49%. With cellulose as a substrate an elevated temperature of 175 °C was necessary for efficient depolymerization to obtain reasonable HMF yield of 40%.
Conclusions
We have studied several aqueous biphasic systems to efficiently promote the fructose dehydration to HMF under MW irradiation. In comparison to conventional heating, a short-term reaction implementing microwaves was more efficient by greatly reducing the reaction time and consequently, the formation of by-products, especially levulinic acid, leading to higher HMF selectivity and yield. Particularly, the use of bromide salts in aqueous phase and common organic solvent MeCN or lignocellulose-derived GVL as an organic extractive phase were highly advantageous resulting in excellent HMF yields of up to 91% with catalytic amount of HCl (9 mol%) as acid catalyst. Only small decrease in HMF yield occurred when Amberlyst-38(wet) catalyst was applied in the system. Also, the reaction was scalable up to fructose concentration of 50 wt% with only small decrease in HMF selectivity.In contrast to fructose dehydration, the biphasic system with added isomerization catalyst performed better with glucose when GVL and Amberlyst-38(wet) were used in place of MeCN and HCl, resulting in very good HMF yield of 74%. The high HMF yield from glucose was ascribed to microwave heating, which promoted the Lewis acid-catalyzed glucose dehydration by overcoming the rate-determining glucose-to-fructose equilibrium barrier, arising from the presence of additional Brønsted acid to enhance the fructose dehydration step. Additionally, this method proved efficient in one-pot cascade hydrolysis–isomerization–dehydration process to produce HMF from disaccharides without additional strong Brønsted acids, and could be applied to more complex carbohydrates such as cellulose. In terms of HMF yields the reported results are, to our knowledge, the best fructose and glucose dehydration results achieved in mono or biphasic aqueous media, and comparable to the best of those obtained in high-boiling organic solvents and ionic liquids.4
Experimental
General experimental details
All solvents, carbohydrates, CrCl3·6H2O, AlCl3·6H2O, boric acid and sodium borate were purchased from Acros Organics or Sigma-Aldrich and were used as received, except THF which was dried by VAC solvent purification system (Vacuum Atmosphere Systems).HMF, glucose and fructose yields were determined with High Pressure Liquid Chromatography (HPLC). HPLC runs were performed using Agilent 1200 HPLC system equipped with a Phenomenex Rezex ROA (300 × 7.8 mm) column. Sulfuric acid (0.25 mM) in water was used as an eluent at 40 °C with a flow rate 0.35 mL min−1. HMF was detected using UV-detector, whereas fructose and glucose were analyzed using refractive index (RID) detector. The exact yields were calculated from calibration curves prepared for all the compounds from commercially available reagents using six different concentrations.
All the reactions were carried out in 2–5 mL glass vials using a Biotage Initiator microwave reactor (2.45 GHz magnetron). The instrument uses one IR sensor to measure temperature of the reaction mixture and adjusts the heating power accordingly. The absorption level was set to “very high” and the reaction mixture was stirred with magnetic stirring at 600 rpm. In all reactions the heating from r.t. to 160 °C took 80 s, after which the desired reaction time was measured.
General procedure for the fructose dehydration experiments in salt(aq)/organic solvent biphasic systems
To a 2–5 mL microwave vial with a magnetic stirring bar containing fructose (0.15 g, 0.83 mmol) was added 1.5 mL of aqueous saturated salt solution (NaCl, NaBr, KCl, KBr, NaI, KI, KF, NH4Cl, NH4Br or NH4I containing the required amount of acid). After dissolution of fructose, desired organic solvent (3 mL) was added, the vial was closed with aluminum/silicone crimp cap and the biphasic solution was heated at 160 °C in the microwave reactor (Biotage Initiator) or in preheated the oil-bath (160 °C) if used. After required time, the vial was immediately cooled down to room temperature and water added to make a total volume of 50 mL. HMF yield and fructose conversion were determined from this solution by HPLC analysis (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution with water, 1 mL).
4 dilution with water, 1 mL).
All MW reactions were performed using synthesis platform (Chemspeed SLT106 Swing Synthesizer) equipped with microwave reactor (Biotage Initiator).
Glucose dehydration in biphasic KBr(aq)–GVL/MeCN/THF system with isomerization and, Amberlyst-38(wet) or HCl catalyst
To a 2–5 mL microwave vial with a magnetic stirring bar containing glucose (0.15 g, 0.83 mmol), 21 mg Amberlyst-38(wet) and 10 mol% of required isomerization catalyst (CrCl3·6H2O, AlCl3·6H2O, boric acid or sodium borate) were added 1.5 mL of aqueous saturated KBr solution. After dissolution of glucose, GVL, THF or MeCN (3 mL) was added, the vial was closed with aluminum/silicone crimp cap and the biphasic solution was heated at 160 °C in the microwave reactor (Biotage Initiator). After required time (1–4 min), the vial was immediately cooled down to room temperature and water added to make a total volume of 50 mL. HMF yield and glucose conversion were determined from this solution by HPLC analysis (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution with water, 1 mL). All reactions were performed using synthesis platform (Chemspeed SLT106 Swing Synthesizer) equipped with microwave reactor (Biotage Initiator).
4 dilution with water, 1 mL). All reactions were performed using synthesis platform (Chemspeed SLT106 Swing Synthesizer) equipped with microwave reactor (Biotage Initiator).
Dehydration of disaccharides and polysaccharides in biphasic (Amberlyst-38-KBr)(aq)–GVL system
To a 2–5 mL microwave vial with magnetic stirring bar containing the desired carbohydrate (0.15 g), 21 mg Amberlyst-38(wet) and 10 mol% of CrCl3·6H2O were added 1.5 mL of aqueous saturated KBr solution. Then GVL was added, the vial closed with aluminum/silicone crimp cap and heated at 160 °C in the microwave reactor (Biotage Initiator). After required time (1–3 min), the vial was immediately cooled down to room temperature and water added to make a total volume of 50 mL. HMF yield and glucose conversion were determined from this solution by HPLC analysis (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dilution with water, 1 mL).
4 dilution with water, 1 mL).
Acknowledgements
Funding from Magnus Ehrnrooth Foundation and TEKES (the Finnish Funding Agency for Technology and Innovation) is gratefully acknowledged.Notes and references
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS PubMed.
- (a) R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed; (b) T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- (a) Z. Huang, W. Pan, H. Zhou, F. Qin, H. Xu and W. Shen, ChemSusChem, 2013, 6, 1063–1069 CrossRef CAS PubMed; (b) C. Aellig and I. Hermans, ChemSusChem, 2012, 5, 1737–1742 CrossRef CAS PubMed.
- S. Dutta, S. De, A. K. Patra, M. Sasidharan, A. Bhaumik and B. Saha, Appl. Catal., A, 2011, 409–410, 133–139 CrossRef CAS.
- C. Moreau, R. Durand, S. Razigade, J. Duhamet, P. Faugeras, P. Rivalier, P. Ros and G. Avignon, Appl. Catal., A, 1996, 145, 211–224 CrossRef CAS.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2010, 111, 397–417 CrossRef PubMed.
- R. M. Musau and R. M. Munavu, Biomass, 1987, 13, 67–74 CrossRef CAS.
- C. Lansalot-Matras and C. Moreau, Catal. Commun., 2003, 4, 517–520 CrossRef CAS.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Green Chem., 2006, 8, 701–709 RSC.
- P. Rivalier, J. Duhamet, C. Moreau and R. Durand, Catal. Today, 1995, 24, 165–171 CrossRef CAS.
- A. J. Crisci, M. H. Tucker, M.-Y. Lee, S. G. Jang, J. A. Dumesic and S. L. Scott, ACS Catal., 2011, 1, 719–728 CrossRef CAS.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- J. N. Chheda and J. A. Dumesic, Catal. Today, 2007, 123, 59–70 CrossRef CAS.
- P. Wrigstedt, J. Keskiväli, M. Leskelä and T. Repo, ChemCatChem, 2015, 7, 501–507 CrossRef CAS.
- T. S. Hansen, J. Mielby and A. Riisager, Green Chem., 2011, 13, 109–114 RSC.
- Q. Cao, X. Guo, J. Guan, X. Mu and D. Zhang, Appl. Catal., A, 2011, 403, 98–103 CrossRef CAS.
- T. Okano, K. Qiao, Q. Bao, D. Tomida, H. Hagiwara and C. Yokoyama, Appl. Catal., A, 2013, 451, 1–5 CrossRef CAS.
- (a) C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC; (b) C. R. Strauss and D. W. Rooney, Green Chem., 2010, 12, 1340–1344 RSC.
- (a) T. S. Hansen, J. M. Woodley and A. Riisager, Carbohydr. Res., 2009, 344, 2568–2572 CrossRef CAS PubMed; (b) X. Qi, M. Watanabe, T. M. Aida and J. R. L. Smith, Green Chem., 2008, 10, 799–805 RSC; (c) J. H. Lu, Y. N. Yan, Y. H. Zhang and Y. Tang, RSC Adv., 2012, 2, 7652–7655 RSC.
- S. De, S. Dutta and B. Saha, Green Chem., 2011, 13, 2859–2868 RSC.
- (a) J. B. Binder, A. V. Cefali, J. J. Blank and R. T. Raines, Energy Environ. Sci., 2010, 3, 765–771 RSC; (b) C. Wang, L. Fu, X. Tong, Q. Yang and W. Zhang, Carbohydr. Res., 2012, 347, 182–185 CrossRef CAS PubMed.
- Y. Roman-Leshkov and J. A. Dumesic, Top. Catal., 2009, 52, 297–303 CrossRef CAS.
- (a) J. S. Luterbacher, J. M. Rand, D. M. Alonso, J. Han, J. T. Youngquist, C. T. Maravelias, B. F. Pfleger and J. A. Dumesic, Science, 2014, 343, 277–280 CrossRef CAS PubMed; (b) M. A. Mellmer, C. Sener, J. M. R. Gallo, J. S. Luterbacher, D. M. Alonso and J. A. Dumesic, Angew. Chem., Int. Ed., 2014, 53, 11872–11875 CrossRef CAS PubMed; (c) E. I. Gürbüz, J. M. R. Gallo, D. M. Alonso, S. G. Wettstein, W. Y. Lim and J. A. Dumesic, Angew. Chem., Int. Ed., 2013, 52, 1270–1274 CrossRef PubMed.
- J. D. Moseley and E. K. Woodman, Energy Fuels, 2009, 23, 5438–5447 CrossRef CAS.
- R. Morschhäuser, M. Krull, C. Kayser, C. Boberski, R. Bierbaum, P. A. Puschner, T. N. Glasnov and C. O. Kappe, Green Process. Synth., 2012, 1, 281–290 Search PubMed.
- B. L. Hayes, Microwave synthesis: chemistry at the speed of light, CEM Pub., Matthews, NC, 2002 Search PubMed.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr, Catal. Commun., 2008, 9, 2244–2249 CrossRef CAS.
- (a) D. J. Braden, C. A. Henao, J. Heltzel, C. C. Maravelias and J. A. Dumesic, Green Chem., 2011, 13, 1755–1765 RSC; (b) A. Aden and T. Foust, Cellulose, 2009, 16, 535–545 CrossRef CAS.
- (a) M. Iannelli, F. Bergamelli, C. M. Kormos, S. Paravisi and N. E. Leadbeater, Org. Process Res. Dev., 2009, 13, 634–637 CrossRef CAS; (b) T. Razzaq and C. O. Kappe, ChemSusChem, 2008, 1, 123–132 CrossRef CAS PubMed; (c) J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794–806 RSC.
- H. B. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- T. Stahlberg, S. Rodriguez-Rodriguez, P. Fristrup and A. Riisager, Chem.–Eur. J., 2011, 17, 1456–1464 CrossRef CAS PubMed.
- (a) V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997–4006 CrossRef CAS PubMed; (b) V. Choudhary, A. B. Pinar, R. F. Lobo, D. G. Vlachos and S. I. Sandler, ChemSusChem, 2013, 6, 2369–2376 CrossRef CAS PubMed.
- (a) Y. J. Pagan-Torres, T. F. Wang, J. M. R. Gallo, B. H. Shanks and J. A. Dumesic, ACS Catal., 2012, 2, 930–934 CrossRef CAS; (b) Y. Yang, C. W. Hu and M. M. Abu-Omar, J. Mol. Catal. A: Chem., 2013, 376, 98–102 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and detailed results. See DOI: 10.1039/c5ra25564c |
| This journal is © The Royal Society of Chemistry 2016 |