The significance of different heating methods on the synthesis of CdS nanocrystals
Huile Jinab,
Liyun Chena,
Aili Liuab,
Lei Guanb,
Dewu Yinb,
Pengsheng Linb,
Shun Wang*b and
Weizhong Jiang*a
aCollege of Materials Science and Engineering, Donghua University, Shanghai, P. R. China 201620. E-mail: jwzh@dhu.edu.cn; Fax: +86-21-67792886; Tel: +86-21-67792886
bNano-materials & Chemistry Key Laboratory, Wenzhou University, Wenzhou, Zhejiang, China 325035. E-mail: shunwang@wzu.edu.cn; Fax: +86-577-86689373; Tel: +86-577-86689373
First published on 11th March 2016
Abstract
Both microwave and conventional oil bath heating approaches are investigated for the fabrication of CdS nanocrystals through the thermolysis of a single source precursor cadmium diethyldithiocarbamate (CED), which allows us to gain further insight into the thermodynamic aspect of the synthesis. The analysis illustrates that nearly monodispersed sea-urchin-shaped CdS nanocrystals are obtained with the microwave method, whereas nanospheres covered by chunky protuberances are achieved with an oil bath approach. Such experiments suggest that microwave heating facilitates the growth of CdS along the [002] direction. In addition, we explored the catalytic activity of CdS in the photodegradation of rhodamine B, in which the CdS prepared through a microwave approach greatly outperformed that prepared through oil bath heating and the commercial CdS nanoparticles. Such enhancement arises from the higher specific surface area and crystallinity of the sea-urchin-shaped nanocrystals.
1. Introduction
The synthesis of nanostructured CdS has attracted a great deal of interest in the last two decades, because this semiconductor has a narrow band gap of 2.4 eV at room temperature, making it suitable for applications in various photoelectric conversions such as in solar cells,1–4 in light emitting diodes for flat-panel displays,5–7 in photocatalytic degradation of contaminants8–13 and in nonlinear optical devices.14–18 Since the size and morphology of nanomaterials greatly affect their concomitant physical and chemical properties, nanocrystalline CdS with different sizes, shapes and dimensionality such as hollow nanospheres,19–22 nanowire23 and cube24 have been fabricated recently. Among the methods developed for the shape-controlled synthesis of CdS products, hydrothermal approach has been employed frequently. For example, He and Gao reported a hydrothermal process to synthesize CdS hierarchical structures using 5-sulfosalicylic acid as additive.25 Wang and co-workers synthesized dendritic CdS nanoarchitectures with cetylpyridinium chloride as a capping reagent.26 Yu's group prepared CdS nanoflowers from cadmium acetate and thiourea via a solvothermal approach in mixed solvents.27 To improve the controllability on the CdS production, a single source precursor was employed by Song and co-workers.28The vast majority of CdS hydrothermal preparation has been carried out in a sealed autoclave placed in an oven. In comparison to such conventional heating method, microwave-assisted decomposition of CdS precursor is underexplored. On the other hand, as a fast, clean and economically viable heating technique, microwave has attracted significant interests in a range of synthetic chemistry, including organic synthesis and inorganic materials preparation.29–34 Besides the localized “hotspot” interaction with reactant molecules, this uniform heating method can also address the problem of heating inhomogeneity encountered in a large scale production, providing a scalable platform for industrial applications. What is more, research has indicated that products synthesized by microwave heating technique tend to have homogeneous size distribution,35–38 high crystallinity39,40 and outstanding catalytic activity,41,42 exhibiting special microwave effect versus the conventional heating.
To synergize the advantages shown by microwave-assisted heating and by single-source precursor decomposition, this research investigated the thermal decomposition of cadmium diethyldithiocarbamate (CED). The commercial reactant molecules CED are precisely defined and thus enable a higher degree of synthetic control. In addition, using single source molecular precursor makes the manipulation of the synthesis simpler, which is much beneficial for large-scale production. To shed light on the importance of thermodynamics versus kinetic control in the crystal growth, heating through oil bath at the atmospheric pressure was also employed. The following results show that nearly monodispersed CdS nanocrystals were achieved with both microwave and oil bath heating processes at the ambient pressure, but their morphology has obvious difference. Experiments on the photodegradation of rhodamine B indicate that CdS nanocrystals prepared through microwave method have much better catalytic performance.
2. Experiment
2.1. Materials
Cadmium diethyldithiocarbamate (CED) was purchased from Zhejiang Ultrafine Powders & Chemicals Co. Ltd. Glycol and CdS nanoparticles were purchased from Aladdin. Rhodamine B (RhB) was purchased from Alfa. Ethanol was purchased from Shanghai Chemical Reagent Co. Deionized water was prepared with a Milli-Q system (18.2 MΩ).2.2. Synthesis of CdS nanocrystal
In a typical procedure 0.05 g (∼0.12 mmol) CED, 48.0 ml glycol and 2.0 ml deionized water were added to a 50 ml round bottom flask. The suspension was stirred continuously at 500 rpm. For microwave-assisted synthesis, the suspension was microwave-heated to 90 °C with a power setting at 200 W and such a process took about 1 minute. The reaction solution was kept at 90 °C for 5 min and then was heated with 500 W power to reach the preselected temperature (e.g., 150, 160 °C etc.), which took about 40 s. The reaction was allowed to proceed at the preselected temperature for 5 minutes or otherwise stated in the context.For the synthesis through conventional heating, the oil bath was first heated to 90 °C and the above 50 ml suspension was kept there for 5 minutes. Then, the oil bath was heated to a preselected temperature such as 160 °C, which took about 13 minutes. After reaching the preset temperature, the reaction was allowed to proceed for another 5 minutes. At the end of the above process the white color suspension turn to yellow, indicating the formation of CdS products. The resulting yellow products, collected through centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 minutes, were washed with deionized water and ethanol several times and were finally dried in vacuum at 50 °C for 1 h. The microwave system used in this study is a Shanghai SINEO MAS-I microwave synthesizer (2450 MHz, maximum power 1000 W).
000 rpm for 15 minutes, were washed with deionized water and ethanol several times and were finally dried in vacuum at 50 °C for 1 h. The microwave system used in this study is a Shanghai SINEO MAS-I microwave synthesizer (2450 MHz, maximum power 1000 W).
2.3. Characterizations
X-ray diffraction spectroscopy (XRD) was performed with a Bruker D8 Advance diffractometer using Cu Kα radiation (λ = 0.15406 nm) and the data was collected in the 2θ range of 20–80° at a step size of 0.02°. Morphology of the as-prepared CdS products was examined by scanning electron microscopy (SEM). Both SEM and Energy dispersive X-ray spectroscopy (EDS) were measured on a FEI Nova Nanosem 200 system operated at an acceleration voltage of 10–15 kV. Selected area electron diffraction (SAED) image and high resolution transmission electron microscopy (HRTEM) were taken on a JEOL 2100F microscope (Japanese Electron Optics Laboratory) at an accelerating voltage of 200 kV. Nitrogen sorption isotherms were measured at the liquid nitrogen temperature 77 K using a Micrometeritics Tristar II 3020, where the samples were degassed for 10 h at 300 °C before the measurements. The specific surface area was calculated using Brunauer–Emmett–Teller (BET) method.2.4. Photocatalytic reactions
For photocatalytic experiments 20.0 mg of CdS catalysts was mixed with 100.0 ml of RhB aqueous solution (C0 = 2 × 10−5 mol l−1) under vigorous stirring. After setting up the reaction system in dark, the reaction solution was irradiated with a Xe arc lamp (300 W, PLS-SXE300, Beijing Trusttech Co. Ltd). The distance between the Xe arc lamp and the liquid level was about 10 cm. The reactor was kept in a water bath to maintain the reaction temperature at 25 °C. At a given irradiation time intervals (20 min), 4.0 ml of the suspension was collected and then centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min) to separate CdS particles, then the clear solution was analyzed with a UV-vis spectrophotometer (UV-1800, SHIMADZU).
000 rpm for 5 min) to separate CdS particles, then the clear solution was analyzed with a UV-vis spectrophotometer (UV-1800, SHIMADZU).
3. Results and discussion
3.1. CdS nanocrystals were synthesized with different heating methods
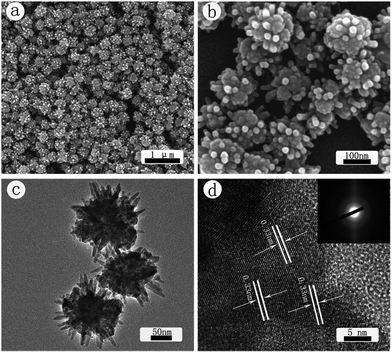
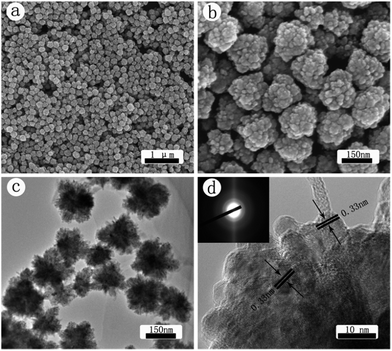
SEM images of the CdS crystals prepared with microwave heating method are shown in Fig. 1(a) and (b). As seen from the low magnification image in Fig. 1(a) nearly monodispersed nanospheres with a spiky surface were obtained at the decomposition temperature of 160 °C. The size of these spheres ranges from 140 to 170 nm. The high resolution SEM image in Fig. 1(b) indicates that the spheres are actually made up of many rods, resembling the morphology of a sea urchin. These nanorods are about 35 nm in length and 10 nm in diameter. TEM image in Fig. 1(c) further confirms that the sphere surface consists of a large number of rods sticking out from a core. The high resolution TEM image in Fig. 1(d) shows the lattice fringes with a spacing of 0.33 nm, which is in good agreement with the d value of the (002) planes of CdS, suggesting that the CdS nanocrystallines, more specifically those nanorods, prefer to grow alone the (002) plane. This observation is the same as reported earlier.43,44 The SAED pattern shown in the inset of Fig. 1(d) indicates that these nanospheres are polycrystalline.CdS crystals prepared with the oil bath heating technique are characterized in Fig. 2. The SEM images in Fig. 2(a) and (b) illustrate that nearly uniform nanospheres with a rough surface are obtained. The size of these nanospheres is about 160 nm. Notably, here the rough surface is made up of many chunky protuberances, as opposed to the rods seen in Fig. 1. The difference in their microstructure can also be seen from the TEM images in Fig. 2(c) and (d). The high-resolution TEM image in Fig. 2(d) shows that the lattice fringes have a spacing of 0.33 nm, which is in good agreement with the d value of the (002) planes of hexagonal CdS. The SAED pattern shown in the inset of Fig. 2(d) indicates that these nanoparticles are polycrystalline. Results in Fig. 1 and 2 highlight the importance of experimental design in the fabrication of CdS nanostructures.
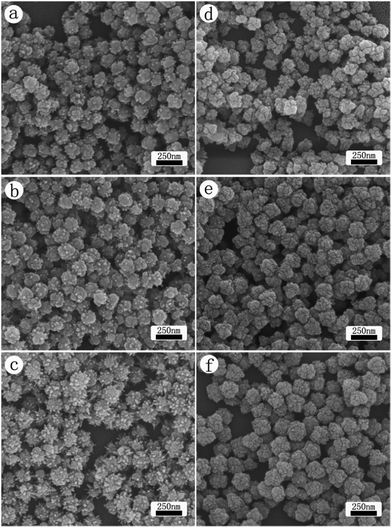
Fig. 3 presents the growth of CdS nanocrystals in time, where the CdS products were harvested at (a, d) 2 min, (b, e) 4 min and (c, f) 6 min are presented. SEM images (a–c) are the CdS products prepared with the microwave heating method, whereas CdS prepared with oil bath heating are presented as (d–f). The results indicate that in the initial stage both heating methods led to the formation of CdS nanoparticles with a rough surface. The size of those particles is around 120 nm, indicating that the decomposition kinetics is rather fast. As the reaction was allowed to progress further, however, the rough surface of these particles grew differently, depending on the heating process. Under oil bath heating, particles appeared to grow evenly, i.e., there is no qualitatively change in the morphology. For the nanoparticles heated with microwaves, on the other hand, those rough spots on the surface grew into rod shape, while the total number of protuberance also increased at the same time. Overall, the size of those spheres grew to about 140 nm at the 4 min mark. As the reaction time was prolonged further, the continuous growth made the difference in their surface morphology more obvious (i.e., the favorite growth of rods in the case of microwave heating versus almost uniform growth of the particle with the oil bath heating). For the oil bath heating system, the SEM image shows nearly identical morphology as seen in Fig. 3(f) after the reaction was allowed to run for another 20 min.
The size distribution of nanoparticles in Fig. 3(a) appears to be narrower than that seen in Fig. 3(d), demonstrating the advantages of microwave method. Such influence likely results from more homogeneous heating and the facilitated generation of nucleation sites. The crystal growth involves the nucleation and the subsequent growth of the nucleus. Rapid formation of the products tends to cause the formation of large number of small particles, followed by the spontaneous aggregation of those small particles. In other word, when the reaction kinetics is fast, the preferential growth of crystal along a specific direction may be hindered, favoring the nanostructure of zero dimension. The observation of nanoparticles in Fig. 3(a) and (d) suggests that the decomposition kinetics of CED is fast at the temperature of 160 °C. Under the similar reaction kinetics, anisotropic growth took place with the microwave method, whereas the morphology seen in (d–f) is close to the situation where the crystal growth is dominated by a fast kinetics. This suggests that microwave plays a unique role in enhancing the growth of CdS along the [002] direction.
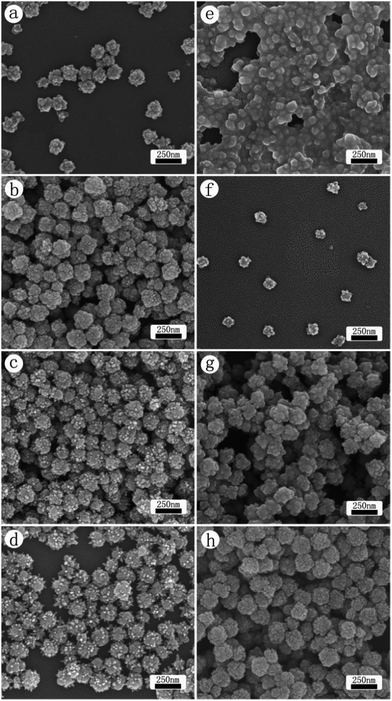
Fig. 4 presents the influence of reaction temperature on the morphology of CdS products: (a–d) microwave method, and (e–h) oil bath heating. The reaction temperature is 110 °C (a, e), 130 °C (b, f), 150 °C (c, g), and 160 °C (d, h). For microwave approach, at the low temperature the reaction only produced nanoparticles with a rough surface. The size of these particles ranges between 80 and 120 nm. As the reaction temperature increased, the growth of nanorod on the surface became more and more obvious, owning to the balance between enhanced preferential growth and the faster kinetics at an elevated temperature. HRTEM characterization such as the one shown in Fig. 1(d) indicates that the favored direction is [002]. Using the conventional heating method, nanoparticles of increasing size were obtained as the reaction temperature was increased from 110 °C to 160 °C, presumably due to the increase of the reaction kinetics at the high temperature. Overall, those nanoparticles from (e) to (h) have qualitatively the same shape and look like emerging from the aggregation of smaller particles combined with the growth of assembled nanoparticles. As the natural crystal structure, hexagonal CdS prefers to grow into 1D such as nanotube. The fact that nanoparticles instead of 1D tubes is obtained with oil bath heating procedure suggests that the reaction kinetics of CED is fast enough to overcome the thermodynamic preference of the CdS crystal growth. At the end, they formed into the nanospheres with rough surface.
The observation that microwave facilitates the directional growth of CdS may be understood from the following. The difference in the electronegativity between Cd and S makes the CdS molecule polar. Under a very high frequency electric field, the polar CdS molecule attempts to follow the field in the same alignment, inducing directional growth. It has been documented that the dipole moment in semiconductor nanocrystals is the governing factor responsible for the assembly of nanocrystal into structures such as nanowires and potentially more complex entities.45 Shao and co-workers reported microwave-templated synthesis of CdS nanotubes in aqueous solution at room temperature.46 Here, the competition between reaction kinetics and the microwave-enhanced direction growth led to the formation of sea-urchin shaped nanocrystal.
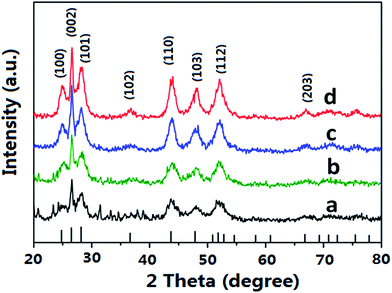
To gain further insight into the role of microwave in the growth of CdS nanostructures, XRD patterns of the CdS products prepared with the two heating methods were recorded in Fig. 5. Those peaks agree with literature data well. The sharp peak at (002) indicates the preferential crystal growth of the CdS products with (002) plane. For the CdS crystalline prepared with microwave approach, the (002) peak intensifies as the reaction temperature increases, owning to the balance between the reaction kinetics and the microwave-facilitated preferential growth. At the same reaction temperature (e.g., 160 °C) the crystallinity of CdS nanospheres synthesized by microwave-assisted heating is higher than that synthesized by oil bath heating, as evidenced by sharp peaks in the XRD spectra. Considering that material's crystallinity is always an important factor which influences photocatalytic activity, we can anticipate different reaction behavior when the above CdS nanocrystals are applied as photocatalysts.
3.2. Photocatalytic degradation of RhB
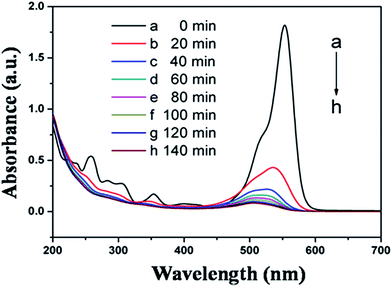
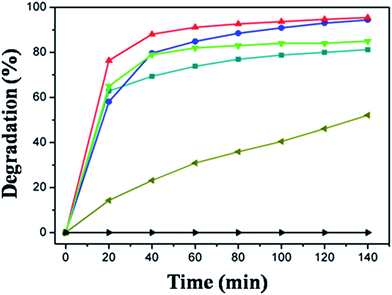
The application of semiconductors in heterogeneous photocatalysis to eliminate various pollutants in aqueous systems has gained significant attention in the last decade. Dye pollutants from the textile industry are an important factor in environmental pollution and RhB is arguably the most important xanthene dye. Shown in Fig. 6 are the absorption spectra of the RhB reaction solution at different reaction stages. There is a significant decrease in the absorption peak after only 20 min, indicating that most of the RhB substrate has photodecomposed in the presence of CdS nanocrystals. According to literature, two reactions may have been involved in the photodecomposition of RhB, i.e., the de-ethylation of RhB and decomposition of the conjugated chromophore structure. Such processes can be characterized respectively by the shift of the maximum absorption band (λmax) and by the change in the absorption maximum (Amax/A0max).47 Usually these two reactions take place simultaneously in the photocatalytic process. In Fig. 6, a blue shift of the maximum absorption band by about 48 nm can be observed after 140 min, indicating the co-occurrence of de-ethylation.To compare the photocatalytic activity of CdS nanocrystals prepared with the two heating methods and at different reaction temperatures, photodecomposition of RhB in the presence of those CdS nanospheres was summarized in Fig. 7. The results indicate that using the CdS catalysts prepared with microwave-assisted synthesis, more than 75% of the RhB had degraded in 20 min. Within the same reaction time period, using the same amount of CdS synthesized through oil bath heating approach, about 65% of the RhB decomposed. This suggests that CdS synthesized by microwave method is about 10% more efficient than those prepared by oil bath. Fig. 7 also shows that the catalytic activity of CdS depend on the temperature at which they were prepared, where the photocatalytic activity of CdS prepared by microwave-method become higher along with their synthesizing temperature. CdS prepared in this research outperformed commercial CdS.
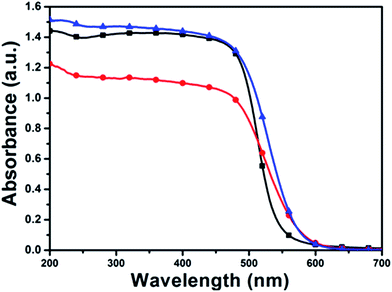
Fig. 8 presents the diffuse-reflectance UV-vis absorption spectra of commercial CdS catalyst, and prepared with microwave-assisted synthesis and oil bath heating approach. Compared with the commercial CdS sample, the CdS catalysts prepared with microwave-assisted synthesis exhibit the more enhanced light absorption intensity in the range of 200–600 nm. The improved light absorption in the visible region suggests that CdS catalysts prepared with microwave-assisted synthesis may enable higher light-driven photocatalytic activity than that of commercial CdS catalyst for given reactions.
Nitrogen sorption isotherms were measured and the surface area was calculated by using the Brunauer–Emmett–Teller (BET) method based on the adsorption data. From the results shown in Table 1, it is clear that BET surface area of the sea urchin-like CdS crystals is significantly higher than the CdS nanospheres with rough surface, presumably because urchin-like CdS nanosphere have many secondary structures (i.e., short nanorods). Meanwhile the BET surface area also grows bigger along with the preparation temperature. The increase in the BET surface area could be responsible for the improvement of the catalytic activity. Notably, as seen in Fig. 7 in the absence of CdS the RhB solution barely has any reactivity. This is because the CdS semiconductors can produce electron–hole pairs under illumination. Most of the photo-generated electrons and holes can transfer to the surface of the crystal when the particle size is small enough and then react with H2O and O2 to produce hydroxyl radical (OH·) which is a strong oxidizing agent to decompose the organic dye.48
| Category | MW 120 °C | MW 150 °C | MW 160 °C | OB 160 °C |
|---|---|---|---|---|
| a MW: microwave heating method; OB: oil bath heating method. | ||||
| BET surface area (m2 g−1) | 17.26 | 94.05 | 175.61 | 30.59 |
4. Conclusion
Using a single-source precursor this study synthesized CdS nanocrystals with two different methods, where sea urchin-like CdS nanocrystals were obtained by microwave-assisted heating technique. Characterizations of the CdS products suggest that microwave does not only provide more uniform heating, but also assists the preferential growth of CdS crystals, leading to higher crystallinity. The achievement of sea urchin nanostructure results from the competition between fast reaction kinetics and microwave-enhanced directional growth. BET measurements illustrate that the CdS nanocrystals prepared with microwave technique has significantly larger specific surface area than the nanocrystals prepared with oil-bath heating method. The large surface area and high crystallinity made the sea urchin-shaped CdS outperformed those prepared with conventional heating method in the photo degradation of RhB, where nearly 80% of the organic dye was decomposed in less than 20 min. More importantly, the idea of using microwave to enhance the photocatalytic activity of CdS nanoparticles may be extended to the fabrication of other semiconductor nanostructures.Acknowledgements
We are grateful for financial supports from NSFC (21471116, 51272182, 51572198 and 21301130), Zhejiang Provincial Natural Science Foundation of China (LY13E020008, LZ15E020002 and LY13B020006).Notes and references
- L. Y. Chang, R. R. Lunt, P. R. Brown, V. Bulovic and M. G. Bawendi, Nano Lett., 2013, 13, 994–999 CrossRef CAS PubMed
.
- M. Shalom, S. Ruhle, I. Hod, S. Yahav and A. Zaban, J. Am. Chem. Soc., 2009, 131, 9876–9877 CrossRef CAS PubMed
.
- R. Yang, D. Wang, L. Wan and D. Wang, RSC Adv., 2014, 4, 22162–22171 RSC
.
- N. R. Yogamalar, K. Sadhanandam, A. C. Bose and R. Jayavel, RSC Adv., 2015, 5, 16856–16869 RSC
.
- T. T. Xuan, J. Q. Liu, R. J. Xie, H. L. Li and Z. Sun, Chem. Mater., 2015, 27, 1187–1193 CrossRef CAS
.
- H. Shen, W. Cao, N. T. Shewmon, C. Yang, L. S. Li and J. Xue, Nano Lett., 2015, 15, 1211–1216 CrossRef CAS PubMed
.
- X. Wang, X. Yan, W. Li and K. Sun, Adv. Mater., 2012, 24, 2742–2747 CrossRef CAS PubMed
.
- A. Kale, Y. Bao, Z. Zhou, P. E. Prevelige and A. Gupta, Nanotechnology, 2013, 24, 045603 CrossRef PubMed
.
- C. Zhang, J. Laic and J. Hu, RSC Adv., 2015, 5, 15110–15117 RSC
.
- Y. Shi, K. Zhou, B. Wang, S. Jiang, X. Qian, Z. Gui, R. Yuen and Y. Hu, J. Mater. Chem. A, 2014, 2, 535–544 CAS
.
- S. S. Boxi and S. Paria, RSC Adv., 2014, 4, 37752–37760 RSC
.
- Z. S. Liu, B. T. Wu, Y. B. Zhu, F. Wang and L. G. Wang, J. Colloid Interface Sci., 2013, 392, 337–342 CrossRef CAS PubMed
.
- D. Jing and L. Guo, J. Phys. Chem. B, 2006, 110, 11139–11145 CrossRef CAS PubMed
.
- S. Gupta, B. R. Mehta and V. R. Satsangi, Nanotechnology, 2012, 23, 355702 CrossRef PubMed
.
- S. S. Talwatkar, Y. S. Tamgadge, A. L. Sunatkari, A. B. Gambhire and G. G. Muley, Solid State Sci., 2014, 38, 42–48 CrossRef CAS
.
- H. S. Kim and K. B. Yoon, Coord. Chem. Rev., 2014, 263–264, 239–256 CrossRef CAS
.
- C. Zheng, W. Chen, S. Cai, X. Xiao and X. Ye, Ceram. Int., 2014, 40, 16245–16251 CrossRef CAS
.
- C. J. Barrelet, A. B. Greytak and C. M. Lieber, Nano Lett., 2004, 4, 1981–1985 CrossRef CAS
.
- W. W. Yu and X. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368–2371 CrossRef CAS
.
- X. Zhang, H. Sun, X. Tao and X. Zhou, RSC Adv., 2014, 4, 31313–31317 RSC
.
- C. Xue, T. Wang, G. Yang, B. Yang and S. Ding, J. Mater. Chem. A, 2014, 2, 7674–7679 CAS
.
- C. Wang, H. Zhang, Z. Lin, X. Yao, N. Lv, M. Li, H. Sun, J. Zhang and B. Yang, Langmuir, 2009, 25, 10237–10242 CrossRef CAS PubMed
.
- G. Li, Y. Jiang, Y. Zhang, X. Lan, T. Zhai and G. Yi, J. Mater. Chem. C, 2014, 2, 8252–8258 RSC
.
- T. I. Levchenko, C. Kübel, D. Wang, B. K. Najafabadi, Y. Huang and J. F. Corrigan, Chem. Mater., 2015, 27, 3666–3682 CrossRef CAS
.
- X. He and L. Gao, Mater. Lett., 2009, 63, 995–997 CrossRef CAS
.
- D. Wang, D. Li, L. Guo, F. Fu, Z. Zhang and Q. Wei, J. Phys. Chem. C, 2009, 113, 5984–5990 CAS
.
- W. T. Yao, S. H. Yu, S. J. Liu, J. P. Chen, X. M. Liu and F. Q. Li, J. Phys. Chem. B, 2006, 110, 11704–11710 CrossRef CAS PubMed
.
- C. Song, Y. Zhang, L. Jiang and D. Wang, Sci. Adv. Mater., 2012, 4, 1096–1102 CrossRef CAS
.
- M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44, 469–478 CrossRef CAS PubMed
.
- M. Baghbanzadeh, L. Carbone, P. D. Cozzoli and C. O. Kappe, Angew. Chem., Int. Ed., 2011, 50, 11312–11359 CrossRef CAS PubMed
.
- A. K. Rathia, M. B. Gawandea, R. Zborila and R. S. Varma, Coord. Chem. Rev., 2015, 291, 68–94 CrossRef
.
- M. B. Gawande, S. N. Shelke, R. Zboril and R. S. Varma, Acc. Chem. Res., 2014, 47, 1338–1348 CrossRef CAS PubMed
.
- S. Zhong, H. Jing, Y. Li, S. Yin, C. Zeng and L. Wang, Inorg. Chem., 2014, 53, 8278–8286 CrossRef CAS PubMed
.
- C. O. Kappe and D. Dallinger, Mol. Diversity, 2009, 13, 71–193 CrossRef CAS PubMed
.
- J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791–15800 CrossRef CAS PubMed
.
- F. F. Munoz, L. M. Acuna, C. A. Albornoz, A. G. Leyva, R. T. Baker and R. O. Fuentes, Nanoscale, 2015, 7, 271–281 RSC
.
- M. Huang, G. Dong, C. Wu and L. Guan, Int. J. Hydrogen Energy, 2014, 39, 4266–4273 CrossRef CAS
.
- A. Pein, M. Baghbanzadeh, T. Rath, W. Haas, E. Maier, H. Amenitsch, F. Hofer, C. O. Kappe and G. Trimmel, Inorg. Chem., 2011, 50(1), 193–200 CrossRef CAS PubMed
.
- Z. Li, R. Teng, S. Zheng, Y. Zhang, T. Huang and G. Lu, J. Cryst. Growth, 2014, 406, 104–110 CrossRef CAS
.
- N. Dahal, S. García, J. Zhou and S. M. Humphrey, ACS Nano, 2012, 6(11), 9433–9446 CrossRef CAS PubMed
.
- S. Barth, M. S. Seifner and J. Bernardi, Chem. Commun., 2015, 51, 12282–12285 RSC
.
- R. J. Pan, Y. C. Wu, Q. P. Wang and Y. Hong, Chem. Eng. J., 2009, 153, 206–210 CrossRef CAS
.
- J. P. Ge and Y. D. Li, Adv. Funct. Mater., 2004, 14(2), 157–162 CrossRef CAS
.
- J. S. Jang, U. A. Joshi and J. S. Lee, J. Phys. Chem. C, 2007, 111, 13280–13287 CAS
.
- S. Shanbhag and N. A. Kotov, J. Phys. Chem. B, 2006, 110, 12211–12217 CrossRef CAS PubMed
.
- M. W. Shao, F. Xu, Y. Peng, J. Wu, Q. Li, S. Y. Zhang and Y. T. Qian, New J. Chem., 2002, 26, 1440–1442 RSC
.
- P. Lei, C. Chen, J. Yang, W. Ma, J. Zhao and L. Zang, Environ. Sci. Technol., 2005, 39, 8466–8474 CrossRef CAS PubMed
.
- Y. Tak, H. Kim, D. Lee and K. Yong, Chem. Commun., 2008, 4585–4587 RSC
.
| This journal is © The Royal Society of Chemistry 2016 |