Adsorption of berberine hydrochloride onto mesoporous carbons with tunable pore size†
Yin Li*ab,
Xiuyang Lub,
Ruiqin Yanga,
Weijian Tonga,
Lijun Xua,
Lucas de Bondelonb,
Hongpeng Wanga,
Ju Zhua and
Qing Gea
aZhejiang Provincial Key Lab for Chem & Bio Processing Technology of Farm Products, School of Biological and Chemical Engineering, Zhejiang University of Science and Technology, Hangzhou 310023, China. E-mail: cherryli1986@126.com; Tel: +86-571-85070380
bKey Laboratory of Biomass Chemical Engineering of Ministry of Education, Zhejiang University, Hangzhou 310027, China
First published on 11th March 2016
Abstract
Eleven mesoporous carbon adsorbents were synthesized and characterized, and the adsorption properties of berberine hydrochloride on these adsorbents were investigated. The mesoporous carbon adsorbents have high BET specific surface areas (1051.2 to 1554.9 m2 g−1), large pore volumes (1.42 to 2.31 m3 g−1), and their average pore radii range from 1.53 nm to 3.28 nm. The adsorption capacities of berberine hydrochloride on these adsorbents strongly depend on the BET specific surface areas and pore volumes of the mesoporous carbons. The mesoporous carbon sample with the highest BET specific surface area was selected as the most promising adsorbent for adsorption of berberine hydrochloride because of its high adsorption capacities (415 mg g−1 at 298 K, 0.10 mg mL−1). The adsorption thermodynamic parameters of berberine hydrochloride on the selected adsorbent were calculated. The adsorption equilibrium of berberine hydrochloride on the selected adsorbent could be established within 240 minutes at 298 K. The dynamic adsorption capacity of berberine hydrochloride on the selected adsorbent is close to the theoretical maximum adsorption capacity; and 72.4% of the adsorbed berberine hydrochloride could be desorbed by a 70% alcohol aqueous solution. The excellent adsorption/desorption properties make the mesoporous carbons promising adsorbents for adsorption of berberine hydrochloride from aqueous solutions.
1. Introduction
Berberine hydrochloride is a quaternary ammonium chloride widely existing in herbal plants. Berberine hydrochloride has broad-spectrum antibiotic activity, and other pharmacological effects such as anti-tumor, anti-oxidant, anti-inflammatory properties; it is extensively used in clinic for treatment of gastroenteric discomfort and adjuvant therapy of type 2 diabetes mellitus, hyperlipidemia, and hypertension.1–5 Berberine hydrochloride is generally obtained from extracts of herbal plants, and a series of separation steps such as dissolving, filtration, re-crystallization and column separation are needed for further purification. The conventional approach needs a plenty of heavy work and is difficult to achieve a high purity of berberine hydrochloride due to the large amount of impurities coexisted in herbal plant extracts.6 On the other hand, berberine hydrochloride is poisonous for microorganisms, difficult to be degraded,7 and could become one of the serious pollutants in water. Therefore, effective purification and separation of berberine hydrochloride is essential for efficient use of herbal extracts and treatment of berberine hydrochloride rich wastewater.Adsorption is believed to be one of the most efficient separation processes and has been successfully applied for separation and purification of natural products like berberine hydrochloride from herbal material extracts. Silica gel (SiO2) and alumina (Al2O3) are the most widely used adsorbents for natural product purification, and using other commercial adsorbents such as activated carbon, polymers and minerals6,8–12 for purifying berberine hydrochloride has been studied, however, high adsorption capacity and selectivity are difficult to achieve on these commonly used commercial adsorbents. Mesoporous (2–50 nm) carbons have the properties of tunable pore structures, high specific surface areas, large pore volumes, high thermal stability and good chemical stability,13,14 which could favor the adsorption of natural products like berberine hydrochloride, and these materials have been applied as adsorbents for removal of pollutants such as aromatic compounds,14–16 organic dyes,17,18 heavy metal ions19–21 and radioactive substances22 from wastewater and purification of biomolecules from aqueous solutions.23,24 Organic–organic self-assembly route has been widely applied to synthesize organic nanostructures and carbon materials including mesoporous carbons.25–28 In the organic–organic self-assembly method for mesoporous carbon synthesis, a thin film containing amphiphilic block copolymers as soft template and suitable carbon precursors is synthesized from the solvent evaporation induced self-assembly of organic molecules, mesoporous carbon is produced after polymerization and carbonization. Pluronic F127 and P123 are two triblock copolymers commonly used as soft templates.25,29–31 From our previous study, mesoporous carbons have been proven to be effective adsorbents for adsorption of berberine hydrochloride from aqueous solutions, and their adsorption properties for berberine hydrochloride heavily depend on their pore textural properties.32,33 However, further experimental data about the relationship between synthesis conditions of mesoporous carbons and their adsorption/desorption properties for berberine hydrochloride is necessary for identifying suitable mesoporous carbon adsorbents.
The main objective of this work was to explore the adsorption equilibrium, kinetics and column breakthrough of berberine hydrochloride on mesoporous carbons with various pore textures to identify suitable mesoporous carbon adsorbents and to develop an efficient process for separation and purification of berberine hydrochloride using mesoporous carbons. Eleven mesoporous carbon adsorbents with different precursor compositions and carbonization temperatures were synthesized, characterized, and tested for adsorption of berberine hydrochloride from aqueous solutions. The relationship between synthesis conditions and pore textural properties of the mesoporous carbons, and the impacts of pore textural properties on adsorption properties for berberine hydrochloride were discussed, and suitable mesoporous carbon adsorbent was identified. These results obtained are essential for selecting and applying the mesoporous carbons for adsorptive purification of natural products like berberine hydrochloride from herbal materials and effective removal of pollutants like berberine hydrochloride from wastewater.
2. Experimental
2.1 Materials
The triblock copolymer Pluronic P123 and tetraethyl orthosilicate (TEOS, >99% purity) were provided by Sigma-Aldrich, berberine hydrochloride (C20H18ClNO4, >98% purity) was obtained by Shanghai Darui Finechem Ltd. (Shanghai, China), AR grade phenol, HF, HCl, NaOH, ethanol and formaldehyde solution (36.5–38 wt%) were purchased from Sinopharm Chemical Reagent Co., Ltd. All these materials were used as received.The molecular structure of berberine hydrochloride as well as its molecular size and optimized molecular structure model performed by the Gaussian 09 software package were listed in Table S1 in the ESI.†
2.2 Synthesis of the mesoporous carbon adsorbents
The mesoporous carbon adsorbents were synthesized via an organic–organic self-assembly method as described previously18 with a small modification. The synthesis of all the carbon samples was done in parallel and finished in one week.A soluble phenolic resin (resol) was produced from phenol and formaldehyde solution under alkaline conditions using the method described in our previous work32 and the resultant product was dissolved in ethanol to obtain a 20 wt% resol ethanolic solution.
The synthesis of mesoporous carbon adsorbents was carried out using the preformed resol solution as a carbon precursor, and tetraethyl orthosilicate (TEOS) and triblock copolymer P123 as templates in an ethanol solution. The synthesis compositions were in the range of phenol/formaldehyde/P123/TEOS (molar ratio) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.021
0.021![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0–1
1.0–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044
0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4. In a typical synthesis process, 1.8580 mL of tetraethyl orthosilicate (TEOS) and 1.0005 mL of 0.2 mol L−1 HCl aqueous solution were added into 5.0570 mL of ethanol, after stirring for 5 h at room temperature, 5.0 g of preformed resol solution, 1.0667 g of P123 and 10.1140 mL of ethanol were then added into it, and the mixture was stirred until P123 was completely dissolved. Afterwards, the solution was transferred into a plate to evaporate ethanol at room temperature, then held at 100 °C in air atmosphere for 24 h. The transparent film obtained was calcined under nitrogen atmosphere at 350 °C for 5 h first to remove the triblock copolymer template, then carbonized at 700–900 °C for 4 h, and the ramping rate was 5 °C min−1. The product was soaked in a 10 wt% HF solution for 24 h at room temperature to remove silica component. The template-free carbon product was then filtered, washed with distilled water, dried, and crushed into powders, and the carbon powder obtained with particle size between 0.18 and 0.45 mm was ready for use.
1.4. In a typical synthesis process, 1.8580 mL of tetraethyl orthosilicate (TEOS) and 1.0005 mL of 0.2 mol L−1 HCl aqueous solution were added into 5.0570 mL of ethanol, after stirring for 5 h at room temperature, 5.0 g of preformed resol solution, 1.0667 g of P123 and 10.1140 mL of ethanol were then added into it, and the mixture was stirred until P123 was completely dissolved. Afterwards, the solution was transferred into a plate to evaporate ethanol at room temperature, then held at 100 °C in air atmosphere for 24 h. The transparent film obtained was calcined under nitrogen atmosphere at 350 °C for 5 h first to remove the triblock copolymer template, then carbonized at 700–900 °C for 4 h, and the ramping rate was 5 °C min−1. The product was soaked in a 10 wt% HF solution for 24 h at room temperature to remove silica component. The template-free carbon product was then filtered, washed with distilled water, dried, and crushed into powders, and the carbon powder obtained with particle size between 0.18 and 0.45 mm was ready for use.
2.3 Characterization of the mesoporous carbons
N2 adsorption–desorption isotherms of the mesoporous carbon adsorbents were measured at 77 K on a sorptometer Quantachrome Autosorb iQ, the carbon samples were degassed under vacuum at 573 K for 6 h prior to the measurement. BET specific surface areas, pore diameter distributions and pore volumes were calculated by using the Autosorb-IQ built-in software ASIQwin v2.0. Transmission electron microscopy (TEM, Joel JEM-2100F) was used to image the mesoporous structures in the OMC sample. The FT-IR spectroscopy was obtained from a PerkinElmer Spectrum 400 FT-IR/FT-NIR spectrometer.2.4 Adsorption isotherms
0.0200 g of the mesoporous carbon adsorbent was accurately weighed and mixed with 100 mL of berberine hydrochloride aqueous solution with a known initial concentration C0 (mg mL−1), and five different initial concentrations were set for each isotherm curve. The mixture was shaken at 298, 308 and 318 K at a constant speed of 200 rpm for 9 h. The final concentration of berberine hydrochloride Ce (mg mL−1) was determined with a Metash UV-5500PC UV-visible spectrophotometer at 345 nm, and the adsorption capacity of berberine hydrochloride on the mesoporous carbon adsorbent Qe (mg g−1) was calculated as:| Qe = (C0 − Ce)V/W | (1) |
2.5 Adsorption kinetics
0.0700 g of the selected mesoporous carbon adsorbent was weighed and introduced into 250 mL of 0.4 mg mL−1 berberine hydrochloride aqueous solution, the mixture was then continuously shaken at 298, 308 and 318 K until the adsorption equilibrium was established. 0.5 mL of the solution was withdrawn at a preset interval during shaking process, the concentration of berberine hydrochloride at the contact time t Ct (mg mL−1) was analyzed, and the corresponding adsorption capacity Qt (mg g−1) was calculated as:| Qt = (C0 − Ct)V/W | (2) |
2.6 Dynamic adsorption and desorption
About 3 mL of the selected mesoporous carbon adsorbent was densely packed into a glass column with an inner diameter of 10 mm, and continuously rinsed by de-ionized water before the dynamic experiment. A berberine hydrochloride aqueous solution with an initial concentration of 0.8 mg mL−1 was passed through the mesoporous carbon column at a flow rate of 14 BV per h (0.7 mL min−1, BV means bed volume, 1 BV = 3 mL). The effluent from the column was collected and the concentration of berberine hydrochloride, C (mg mL−1), was analyzed until it was close to the initial concentration.After that, the mesoporous carbon column was first rinsed with 6 mL of de-ionized water, then 70% of ethanol aqueous solution (v/v) was used to desorb berberine hydrochloride from the mesoporous carbon column, the flow rate was set at 6.4 BV per h, the effluent was collected and the concentration of berberine hydrochloride was analyzed until the concentration approached zero.
3. Results and discussion
3.1 Pore textures of the mesoporous carbons
The synthesis conditions and the main pore textural properties including BET surface areas, pore volumes and average pore diameters of the mesoporous carbon adsorbents are listed in Table 1. The N2 adsorption and desorption isotherms and pore diameter distributions calculated from the desorption isotherm branches of the mesoporous carbons are shown in Fig. S1 and S2, respectively, in ESI.† Overall, the pore textural properties of the these mesoporous carbon samples are consistent with those of mesoporous carbon materials reported in literatures and our previous work.18,33 The BET specific surface areas of the mesoporous carbon adsorbents were in the range of 1051.2 to 1554.9 m2 g−1, and their pore volumes were measured to be between 1.42 and 2.31 cm3 g−1. The average pore radii of the mesoporous carbon samples were measured to be between 1.53 to 3.28 nm, and mesopores with the radius distributions between 1 and 10 nm are the most predominant pores in the samples. Of all the 11 samples, sample 8-1 with the molar compositions of phenol/formaldehyde/P123/TEOS (molar ratio) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044
0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 showed the highest BET specific surface area of 1554.9 m2 g−1, and sample 9 with the molar compositions of phenol/formaldehyde/P123/TEOS = 1
1.2 showed the highest BET specific surface area of 1554.9 m2 g−1, and sample 9 with the molar compositions of phenol/formaldehyde/P123/TEOS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044
0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 displayed the largest pore volume of 2.31 cm3 g−1.
1.4 displayed the largest pore volume of 2.31 cm3 g−1.
| Samples | Molar ratio of (phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formaldehyde formaldehyde![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P123 P123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEOS) TEOS) |
Carbonization temperature (°C) | BET specific surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore radius (nm) |
|---|---|---|---|---|---|
| Sample 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.021 0.021![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
900 | 1106.0 | 1.49 | 1.70 |
| Sample 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.021 0.021![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
900 | 1051.2 | 1.42 | 1.91 |
| Sample 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.021 0.021![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
900 | 1347.8 | 2.04 | 1.71 |
| Sample 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.033 0.033![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
900 | 1224.3 | 1.58 | 1.71 |
| Sample 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.033 0.033![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
900 | 1365.8 | 1.91 | 2.44 |
| Sample 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.033 0.033![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
900 | 1537.0 | 1.92 | 1.70 |
| Sample 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044 0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
900 | 1386.6 | 1.93 | 1.70 |
| Sample 8–1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044 0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
900 | 1554.9 | 2.03 | 1.53 |
| Sample 8–2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044 0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
800 | 1526.7 | 1.80 | 2.81 |
| Sample 8–3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044 0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
700 | 1292.9 | 1.74 | 3.28 |
| Sample 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.044 0.044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
900 | 1533.5 | 2.31 | 1.70 |
The BET specific surface areas, pore volumes and average pore radii of the 9 mesoporous carbon samples with various synthesis compositions were plotted in Fig. 1a–c. As shown in Fig. 1a and b, the BET surface areas and pore volumes of the mesoporous carbon samples showed significant differences and a similar tendency, the increase of the TEOS composition as well as the P123 amount has a positive effect on both the BET surface areas and pore volumes, and large BET surface areas and large pore volumes were obtained from the mesoporous carbon samples with a higher TEOS composition and larger P123 amount. However, sample 6, sample 8-1 and sample 9 with the highest P123 amounts and TEOS compositions showed similar BET surface areas, which indicated that a too high P123 and TEOS composition could not further improve the surface areas of the mesoporous carbons. On the other hand, the median pore radii of the mesoporous carbon samples don't present a linear correlation with neither their TEOS compositions nor P123 amounts. These results might attribute to the different effects from the hard template and soft template on the pore textural properties of mesoporous carbons.33 TEOS was used as a hard template, large amount of TEOS has a positive effect on pore forming to obtain large surface areas and pore volumes for mesoporous carbons.33 P123 was used as a soft template, large amount of P123 is favourable for expanding pores to obtain large surface areas and pore volumes.33 In the range of the synthesis compositions for mesoporous carbons in this study, the compositions of both the two templates did not show significant effects on the pore radii of the mesoporous carbons (Fig. 1c).
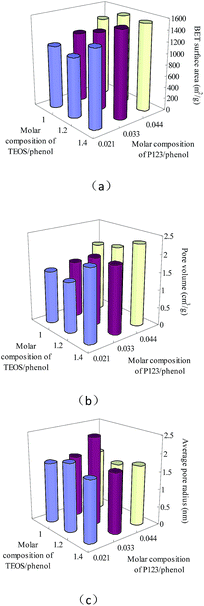 | ||
| Fig. 1 The pore textural properties of the mesoporous carbons with different synthesis compositions: (a) BET specific surface area; (b) pore volume; (c) average pore radius. | ||
On the other hand, the mesoporous carbons studied in this work have lower BET specific surface areas (1051.2 to 1554.9 m2 g−1) and smaller pore volumes (1.42 to 2.31 cm3 g−1) than those of the mesoporous carbons reported in our previous work (BET surface areas of 1590.3 to 2193.5 m2 g−1 and pore volumes of 1.72 to 2.56 m3 g−1) with F127 as a soft template at similar synthesis conditions, while their pore sizes are similar.33 F127 has a larger molecular weight (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) and a longer chain length than those of P123 (molecular weight of 5800), which might lead to a stronger pore expanding effect than P123 to obtain larger surface areas and pore volumes, on the other side, similar pore sizes of the mesoporous carbons with either P123 or F127 as soft templates might attribute to the combination of the pore forming effect from TEOS and the pore expanding effect from F127 or P123.
600) and a longer chain length than those of P123 (molecular weight of 5800), which might lead to a stronger pore expanding effect than P123 to obtain larger surface areas and pore volumes, on the other side, similar pore sizes of the mesoporous carbons with either P123 or F127 as soft templates might attribute to the combination of the pore forming effect from TEOS and the pore expanding effect from F127 or P123.
From the BET specific surface areas, pore volumes and average pore radii of the mesoporous carbon sample 8-1, 8-2 and 8-3 (Table 1) with same synthesis compositions, approximately linear correlations were observed between the pore textural properties and the carbonization temperatures, and it can be found out that with the increase of carbonization temperature, the BET specific surface area as well as the pore volume of the mesoporous carbon samples increased, smaller pore radius was obtained. These results indicated that in a certain range, high carbonization temperature is favourable for producing a more cross-link mesoporous carbon to obtain larger surface area, larger pore volume but narrower pores.
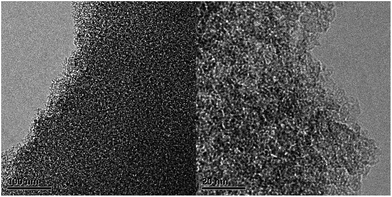
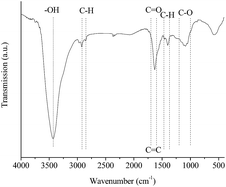
High-resolution TEM images (Fig. 2) of sample 8-1 revealed an amorphous structure of the mesoporous carbon and homogeneous pores throughout the whole sample, these are similar to the reports in the literature.34 The FT-IR spectroscopy of sample 8-1 was displayed in Fig. 3, few functional groups were observed on the surface of the carbon sample 8-1, which might due to the high temperature calcination process under nitrogen atmosphere. The adsorption band observed at 3432 cm−1 is assigned to O–H stretching vibrations, the peaks between 2850 cm−1 and 2920 cm−1 and the band between 1370 cm−1 and 1470 cm−1 could be attribute to C–H groups, the adsorption peaks between 2850 cm−1 and 2920 cm−1 represent C–H stretching vibrations, the peak between 1600 cm−1 and 1700 cm−1 is attribute to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups and aromatic C
O functional groups and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and the week band between 1000 cm−1 and 1200 cm−1 represents C–O bending vibrations, these results suggested that a small amount of O-containing functional groups are present in the mesoporous carbon sample.
C, and the week band between 1000 cm−1 and 1200 cm−1 represents C–O bending vibrations, these results suggested that a small amount of O-containing functional groups are present in the mesoporous carbon sample.
3.2 Adsorption isotherms of berberine hydrochloride
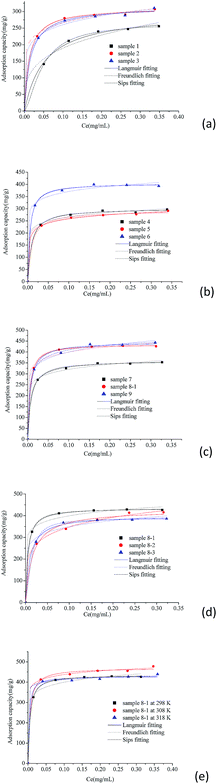
Adsorption isotherms of berberine hydrochloride on the mesoporous carbon adsorbents are displayed in Fig. 4. It can be found from Fig. 4 that the equilibrium adsorption capacities of berberine hydrochloride on the mesoporous carbon samples are in the range from about 195 to 415 mg g−1, at an equilibrium concentration of 0.10 mg mL−1 and adsorption temperature of 298 K. Generally, the adsorption of berberine hydrochloride on all the mesoporous carbons studied in this work is effective, and the adsorption capacities strongly depend on the pore textural properties of the carbon samples. BET specific surface area and pore volume are known to be two predominant factors influencing adsorption processes in aqueous solution. The mesoporous carbons studied in this work have high BET surface areas (1051.2 to 1554.9 m2 g−1), large pore volumes (1.42 to 2.31 cm3 g−1), which could result in the effective adsorption for berberine hydrochloride. Moreover, mesopores with the radius distributions between 1 and 10 nm are the most predominant pores in the mesoporous carbons samples, and the average pore radii are between 1.53 and 3.28 nm which are big enough for the adsorption of berberine hydrochloride with the molecular size of 1.32 nm × 0.66 nm × 0.32 nm.35 However, these adsorption capacity values are less than that of the mesoporous carbons with F127 as a soft template obtained in our previous work33 at similar conditions, which might because of the even higher BET specific surface areas (1590.3 to 2193.5 m2 g−1) and larger pore volumes (1.72 to 2.56 m3 g−1) of the mesoporous carbons with F127 as a soft template.As shown in Fig. 4a–d, sample 8-1, 9 and 6 displayed relatively higher adsorption capacities for berberine hydrochloride at 298 K, while sample 1 served the lowest adsorption capacity, and the adsorption capacities of all the 11 mesoporous carbon samples are generally consistent with the order of their BET surface areas and pore volumes. The mesoporous carbon sample 5, 6 and 7 have similar pore volumes, and their adsorption capacities for berberine hydrochloride were measured to be 261, 380 and 328 mg g−1, respectively, at the equilibrium concentration of 0.10 mg mL−1, which agree the order of their BET surface areas. Additionally, adsorption capacities of berberine hydrochloride on the mesoporous carbon sample 6, 8-2 and 9 with similar BET surface areas were determined to be 380, 347, and 400 mg g−1 at the equilibrium concentration of 0.10 mg mL−1, respectively, which exhibit the same order of their pore volumes. These results further confirmed the dominant roles and positive impacts of the BET surface area and pore volume on the adsorption for berberine hydrochloride from aqueous solutions.
From the isotherms of berberine hydrochloride on the mesoporous carbon sample 8-1 at 298, 308 and 318 K shown in Fig. 4e, it can be found out that the adsorption capacities of berberine hydrochloride increased with increasing temperature from 298 K to 308 K, however, the adsorption amounts decreased with further increase of feed temperature from 308 K to 318 K, and the mesoporous carbon sample displayed similar adsorption capacities for berberine hydrochloride at 298 K and 318 K, which might suggest a complex adsorption mechanism (the combination of physical adsorption and weak chemical adsorption) of berberine hydrochloride on the mesoporous carbon.
Langmuir, Freundlich and Sips isotherm models were employed to analyze the adsorption isotherm data of berberine hydrochloride on the mesoporous carbons, they can be represented as:
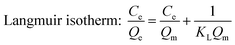 | (3) |
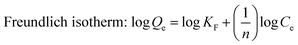 | (4) |
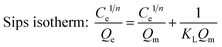 | (5) |
The calculated parameters such as Qm, KL, KF and 1/n along with the correlation coefficients R2 of Langmuir, Freundlich and Sips equations are listed in Table 2. It is found that all the three models fit the isotherms of berberine hydrochloride on the mesoporous carbons very well, and Sips equation is slightly better than the Langmuir and Freundlich models for describing the adsorption behavior of berberine hydrochloride. However, Qm values from the Sips model for sample 8-1 at 308 K, 318 K and sample 8-2 at 298 K were considerably higher than the experimental adsorption capacities, which might due to the adsorbate–adsorbate interactions in the adsorption process according to the assumption of the model.39 These results indicate a complex adsorption mechanism of berberine hydrochloride on the mesoporous carbons rather than a simple monolayer adsorption on the active adsorption sites with homogeneous distribution.
| Mesoporous carbon samples | Langmuir model | Freundlich model | Sips model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm/(mg g−1) | KL /(mL mg−1) | R2 | KF/[(mg g−1) (mL mg−1)1/n] | 1/n | R2 | Qm/(mg g−1) | KL (mL mg−1)1/n | 1/n | R2 | |
| Sample 1 at 298 K | 297.6 | 19.35 | 0.997 | 355.0 | 0.2722 | 0.984 | 266.6 | 101.9 | 1.509 | 0.999 |
| Sample 2 at 298 K | 311.8 | 78.89 | 0.999 | 351.1 | 0.1204 | 0.996 | 314.1 | 64.16 | 0.9457 | 0.999 |
| Sample 3 at 298 K | 313.9 | 63.64 | 0.997 | 358.8 | 0.1392 | 0.996 | 340.0 | 16.97 | 0.6672 | 0.998 |
| Sample 4 at 298 K | 301.4 | 108.1 | 0.999 | 331.9 | 0.09486 | 0.996 | 303.2 | 85.79 | 0.9398 | 0.999 |
| Sample 5 at 298 K | 290.0 | 109.3 | 0.998 | 319.9 | 0.09458 | 0.999 | 368.4 | 5.01 | 3.090 | 0.999 |
| Sample 6 at 298 K | 404.3 | 192.7 | 0.999 | 447.0 | 0.08101 | 0.995 | 406.9 | 137.3 | 0.3237 | 0.999 |
| Sample 7 at 298 K | 360.1 | 123.8 | 0.999 | 402.4 | 0.09875 | 0.997 | 370.2 | 50.61 | 0.7813 | 0.999 |
| Sample 8-1 at 298 K | 436.4 | 197.7 | 0.999 | 487.6 | 0.08661 | 0.994 | 432.8 | 349.4 | 1.129 | 0.999 |
| Sample 8-1 at 308 K | 469.3 | 194.6 | 0.998 | 499.8 | 0.05833 | 0.999 | 1007 | 0.9742 | 0.1032 | 0.999 |
| Sample 8-1 at 318 K | 429.4 | 345.9 | 0.998 | 446.5 | 0.03479 | 0.999 | 940.7 | 0.8995 | 0.06168 | 0.999 |
| Sample 8-2 at 298 K | 427.2 | 61.54 | 0.992 | 520.2 | 0.1765 | 0.998 | 907.8 | 349.4 | 0.2861 | 0.999 |
| Sample 8-3 at 298 K | 401.1 | 99.73 | 0.999 | 453.4 | 0.1134 | 0.999 | 386.8 | 880.1 | 1.561 | 0.999 |
| Sample 9 at 298 K | 445.6 | 155.2 | 0.997 | 514.0 | 0.1090 | 0.997 | 497.2 | 15.49 | 0.5192 | 0.999 |
Generally, 1/n ≤ 1, stands for a preferential adsorption procedure, which indicates that the adsorption is easy to occur.17,40 All the mesoporous carbon samples have a 1/n value of less than 0.3 for the adsorption of berberine hydrochloride indicating a favorable adsorption of berberine hydrochloride on all the mesoporous carbons.
Three thermodynamic parameters, adsorption enthalpy (ΔH0), adsorption entropy (ΔS) and adsorption free energy (ΔG) of berberine hydrochloride on the mesoporous carbon sample 8-1 were calculated according to the adsorption isotherms of berberine hydrochloride at 298, 308 and 318 K using Langmuir equation.40–42 The calculated values of ΔG at 298, 308 and 318 K are −27.76, −28.65 and −31.10 kJ mol−1, respectively, and the values of ΔS and ΔH0 are 167.1 J mol−1 K−1 and 22.30 kJ mol−1, respectively. It was observed that the ΔG values of berberine hydrochloride at three different temperatures are negative, suggesting that the adsorption of berberine hydrochloride on the mesoporous carbon was thermodynamically spontaneous. Moreover, the positive ΔS value further confirmed the thermodynamical feasibility of the adsorption of berberine hydrochloride on the mesoporous carbon sample. However, the value of ΔH0 is positive, which implied that the adsorption of berberine hydrochloride is endothermic, and similar results were also reported in our previous work and earlier literatures.32,43 The positive ΔH0 value confirmed the complex adsorption mechanism of berberine hydrochloride on the mesoporous carbon, indicated that the adsorption of berberine hydrochloride on the mesoporous carbon was not a totally physical process while a weak chemical adsorption might also be involved. The chemical adsorption of berberine hydrochloride on the mesoporous carbon might attribute to the structure of the berberine hydrochloride molecule and the surface chemistry of the mesoporous carbon. Berberine hydrochloride is an alkaloid salt which exists in an ionic state in its aqueous solution.44 The residual oxygenic functional groups on the surface of the mesoporous carbon could possible adsorb berberine hydrochloride molecules through weak chemical interactions.
3.3 Adsorption kinetics of berberine hydrochloride on the mesoporous carbon
The mesoporous carbon sample 8-1 has a high BET surface area and large pore volume, and exhibited the largest adsorption capacity toward berberine hydrochloride, therefore was selected for the following kinetic and dynamic adsorption breakthrough tests for berberine hydrochloride.The adsorption kinetics of berberine hydrochloride on the mesoporous carbon sample 8-1 from aqueous solutions at 298 K, 308 K and 318 K were displayed in Fig. 5. The adsorption of berberine hydrochloride on the mesoporous carbon was shown to be fast, and shorter time was required for establishing adsorption equilibrium at higher temperature. At 298 K, the adsorption rate of berberine hydrochloride was quite fast in the first hour and about 77% of the equilibrium adsorption capacity had been reached, and the final equilibrium was arrived within 240 min. These results exhibited the good kinetic properties of the mesoporous carbon, and implied the efficiency of the mesoporous carbon for adsorptive removal or purification of berberine hydrochloride from aqueous solutions. However, as compared with the time required for reaching the adsorption equilibrium for berberine hydrochloride on sample 8-1 and the mesoporous carbon sample reported in our previous work with F127 as a soft template at 298 K, even shorter time (90 min) was needed for berberine hydrochloride on that mesoporous carbon sample reported in our previous work.32 The mesoporous carbon sample reported in our previous work with F127 as a soft template has an average pore diameter of 4.21 nm,32 which is larger than that of the mesoporous carbon sample 8-1 in this study, larger pore size is more favourable for the diffusion of berberine hydrochloride in the pores of the mesoporous carbon which might lead to a better kinetic property.
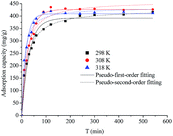 | ||
| Fig. 5 Adsorption kinetics of berberine hydrochloride on the mesoporous carbon sample 8-1 from aqueous solution. | ||
Pseudo-first-order rate equation (Lagergren's rate equation) and pseudo-second-order equation proposed by Ho and McKay,45,46 were applied to fit the adsorption kinetics of berberine hydrochloride on the mesoporous carbon sample 8-1, the two equations are presented as:
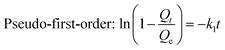 | (6) |
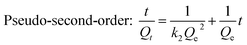 | (7) |
The fitted parameters including k1, k2, Qe, v0 as well as the correlation coefficients R2 from both kinetic equations were summarized in Table 3. It can be found that the pseudo-second-order rate equation is slightly better than the pseudo-first-order rate equation for describing the kinetic data of berberine hydrochloride. Meanwhile, the initial adsorption rate v0 calculated in Table 3 further confirmed the high efficiency of the mesoporous carbon for adsorption of berberine hydrochloride from aqueous solutions at all the three selected temperatures, and the v0 values clearly increased with increasing temperature, which suggested the positive effect of temperature on the adsorption efficiency.
| Temperature/(K) | Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | k1 (min−1) | R2 | Qe (mg g−1) | k2 | v0 (mg g−1 min−1) | R2 | |
| 298 | 391.6 | 3.245 × 10−2 | 0.966 | 428.8 | 1.100 × 10−4 | 20.23 | 0.995 |
| 308 | 426.8 | 3.599 × 10−2 | 0.980 | 461.1 | 1.200 × 10−4 | 25.51 | 0.979 |
| 318 | 409.4 | 5.632 × 10−2 | 0.979 | 435.0 | 2.300 × 10−4 | 43.52 | 0.988 |
3.4 Dynamic adsorption and desorption of berberine hydrochloride
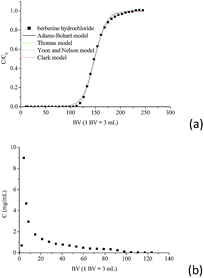
The dynamic adsorption breakthrough curve and desorption curve of berberine hydrochloride on the mesoporous carbon sample 8-1 at room temperature were displayed in Fig. 6(a and b). As shown in Fig. 6a, the breakthrough point (C/C0 = 0.05) and total adsorption volume (C/C0 = 0.95) of berberine hydrochloride from the mesoporous carbon column were measured to be 120 BV and 192 BV, respectively, and the dynamic adsorption capacity calculated from the numerical integration of the breakthrough curve was to be 425.3 mg g−1, which is close to the theoretical maximum adsorption equilibrium capacity (436.4 mg g−1) calculated from the isotherm data of berberine hydrochloride on this sample at 298 K according to Langmuir equation.Various mathematical models have been developed to predict the dynamic adsorption behavior of the column. Adams–Bohart, Thomas, Yoon and Nelson, and Clark models were used to analyze the dynamic adsorption breakthrough data of berberine hydrochloride,39,47–50 they are presented as:
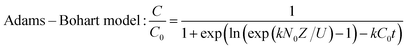 | (8) |
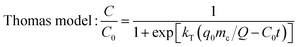 | (9) |
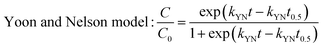 | (10) |
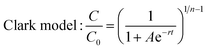 | (11) |
The parameters calculated from the four models together with the correlative coefficients R2 were summarized in Table 4. It can be seen that the correlation coefficients for all the four models exceeded 0.99, which indicated that all the four models are suitable for predicting the dynamic behaviour of berberine hydrochloride to be adsorbed from solution in a fixed mesoporous carbon column bed. The N0 calculated from the Adams–Bohart model was 118.7 mg mL−1 (430.7 mg g−1), and the q0 calculated from the Thomas model was 430.8 mg g−1, both of them were close to the experimental dynamic adsorption capacity, additionally, the t0.5 value from the Yoon and Nelson model was calculated to be 636.0 min, which was also close to the experimental value of 630.0 min. These results further confirmed the validity of these three models and the high adsorption efficacy of the mesoporous carbon adsorbent for berberine hydrochloride.
| Adams–Bohart model | Thomas model | ||||
|---|---|---|---|---|---|
| k (mL min−1 mg−1) | N0 (mg mL−1) | R2 | kT (mL min−1 mg−1) | q0 (mg g−1) | R2 |
| 0.02501 | 118.7 | 0.999 | 0.02501 | 430.8 | 0.999 |
| Yoon and Nelson model | Clark model | ||||
|---|---|---|---|---|---|
| kYN (min−1) | t0.5 (min) | R2 | A | r | R2 |
| 0.02001 | 636.0 | 0.999 | 558.2 | 0.01431 | 0.999 |
As can be seen from Fig. 6b, about 60 bed volume of 70% (v/v) ethanol aqueous solution could elute about 72.4% of berberine hydrochloride from the mesoporous carbon column, and 100 bed volume was needed to reach a desorption ratio of 82.9% for berberine hydrochloride, these results implied that the adsorbed mesoporous carbon adsorbent can be regenerated and reused easily.
4. Conclusions
Eleven mesoporous carbon adsorbents were synthesized, characterized and evaluated their adsorption/desorption properties of berberine hydrochloride from aqueous solutions. The mesoporous carbon adsorbents synthesized in this work exhibit high BET specific surface areas (1051.2 to 1554.9 m2 g−1), large pore volumes (1.42 to 2.31 m3 g−1), and their average pore radii are between 1.53 nm and 3.28 nm. Pore textural properties of the mesoporous carbon adsorbents strongly effects the adsorption of berberine hydrochloride from aqueous solution, and the mesoporous carbon sample with the largest surface area showed the highest adsorption capacity (415 mg g−1) at 298 K and 0.10 mg mL−1 for berberine hydrochloride and was selected as the most promising adsorbent. Moreover, the adsorption rate of berberine hydrochloride on the selected mesoporous carbon is fast, less than 240 min is needed to reach equilibrium at 298 K. The dynamic adsorption tests indicated the effective dynamic adsorption and easy regeneration of the selected mesoporous carbon for berberine hydrochloride. The mesoporous carbons especially the selected carbon sample studied in this work are promising adsorbent materials for adsorptive removal and purification of berberine hydrochloride and similar alkaloids from aqueous solutions.Acknowledgements
This work was partially supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ15B060003), the Foundation of Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Zhejiang University, the Zhejiang Province Research Project of Public Welfare Technology Application (No. 2015C33006), and the Analysis & Test Funding Project of Zhejiang Province (No. 2015C37059).Notes and references
- X. Luo, J. Li, L. Guo, X. Cheng, T. Zhang and Y. Deng, Asian J. Pharm. Sci., 2013, 8, 261–266 CrossRef.
- Y. Wang, Q. Liu, Z. Liu, B. Li, Z. Sun, H. Zhou, X. Zhang, Y. Gong and C. Shao, Mutat. Res., 2012, 734, 20–29 CrossRef CAS PubMed.
- J. Lan, Y. Zhao, F. Dong, Z. Yan, W. Zheng, J. Fan and G. Sun, J. Ethnopharmacol., 2015, 161, 69–81 CrossRef CAS PubMed.
- E. Küpeli, M. Koşar, E. Yeşilada and K. H. C. Başer, Life Sci., 2002, 72, 645–657 CrossRef.
- A. Shirwaikar, A. Shirwaikar, K. Rajendran and I. S. R. Punitha, Biol. Pharm. Bull., 2006, 29, 1906–1910 CAS.
- H. Li, Y. Li, Z. Li, X. Peng, Y. Li, G. Li, X. Tan and G. Chen, Appl. Surf. Sci., 2012, 258, 4314–4321 CrossRef CAS.
- M. Ren, Y. Song, S. Xiao, P. Zeng and J. Peng, Chem. Eng. J., 2011, 169, 84–90 CrossRef CAS.
- Y. Li, J. Huang, J. Liu, S. Deng and X. Lu, J. Chem. Eng. Data, 2013, 58, 1271–1279 CrossRef CAS.
- J. Votruba and M. Flieger, Biotechnol. Lett., 2000, 22, 1281–1285 CrossRef CAS.
- D. X. Wang, A. Sakoda and M. Suzuki, Adsorption, 1999, 5, 97–108 CrossRef CAS.
- G. Rytwo, H. Varman, N. Bluvshtein, T. N. König, A. Mendelovits and A. Sandler, Appl. Clay Sci., 2011, 51, 43–50 CrossRef CAS.
- E. Cohen, T. Joseph, I. Lapides and S. Yariv, Clay Miner., 2005, 40, 223–232 CrossRef CAS.
- J. Górka and M. Jaroniec, J. Phys. Chem. C, 2010, 114, 6298–6303 Search PubMed.
- J. Cai, S. Bennici, J. Shen and A. Auroux, Water, Air, Soil Pollut., 2014, 225, 2088 CrossRef.
- K. Wang, B. Huang, D. Liu and D. Ye, J. Appl. Polym. Sci., 2012, 125, 3368–3375 CrossRef CAS.
- M. Anbia and S. Salehi, Chem. Eng. Res. Des., 2012, 90, 975–983 CrossRef CAS.
- X. Peng, D. Huang, T. Odoom-Wubah, D. Fu, J. Huang and Q. Qin, J. Colloid Interface Sci., 2014, 430, 272–282 CrossRef CAS PubMed.
- X. Zhuang, Y. Wan, C. Feng, Y. Shen and D. Zhao, Chem. Mater., 2009, 21, 706–716 CrossRef CAS.
- G. Zeng, Y. Liu, L. Tang, G. Yang, Y. Pang, Y. Zhang, Y. Zhou, Z. Li, M. Li, M. Lai, X. He and Y. He, Chem. Eng. J., 2015, 259, 153–160 CrossRef CAS.
- B. Acevedo and C. Barriocanal, RSC Adv., 2015, 5, 95247–95255 RSC.
- G. Yang, L. Tang, Y. Cai, G. Zeng, P. Guo, G. Chen, Y. Zhou, J. Tang, J. Chen and W. Xiong, RSC Adv., 2014, 4, 58362–58371 RSC.
- Z.-b. Zhang, Y.-d. Zhou, Y.-H. Liu, X.-h. Cao, Z.-w. Zhou, B. Han, P. Liang and G.-x. Xiong, J. Radioanal. Nucl. Chem., 2014, 302, 9–16 CrossRef CAS.
- A. Vinu, K. Z. Hossian, P. Srinivasu, M. Miyahara, S. Anandan, N. Gokulakrishnan, T. Mori, K. Ariga and V. V. Balasubramanian, J. Mater. Chem., 2007, 17, 1819–1825 RSC.
- Z. Wang, X. Liu, M. Lv and J. Meng, Carbon, 2010, 48, 3182–3189 CrossRef CAS.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, L. Cheng, D. Feng, Z. Wu, Z. Chen, Y. Wan, A. Stein and D. Zhao, Chem. Mater., 2006, 18, 4447–4464 CrossRef CAS.
- Q. Wang, W. Zhang, Y. Mu, L. Zhong, Y. Meng and Y. Sun, Microporous Mesoporous Mater., 2014, 197, 109–115 CrossRef CAS.
- S. Tanaka, N. Nishiyama, Y. Egashira and K. Ueyama, Chem. Commun., 2005, 28, 2125–2127 RSC.
- C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696–3717 CrossRef CAS PubMed.
- C. Liang and S. Dai, J. Am. Chem. Soc., 2006, 128, 5316–5317 CrossRef CAS PubMed.
- W. Libbrecht, F. Deruyck, H. Poelman, A. Verberckmoes, J. Thybaut, J. De Clercq and P. Van Der Voort, Chem. Eng. J., 2015, 259, 126–134 CrossRef CAS.
- J. Jiao, Y. Cai, P. Liu and P. Liu, J. Porous Mater., 2013, 20, 1247–1255 CrossRef CAS.
- Y. Li, B. Yuan, J. Fu, S. Deng and X. Lu, J. Colloid Interface Sci., 2013, 408, 181–190 CrossRef CAS PubMed.
- Y. Li, J. Fu, S. Deng and X. Lu, J. Colloid Interface Sci., 2014, 424, 104–112 CrossRef CAS PubMed.
- B. Yuan, X. Wu, Y. Chen, J. Huang, H. Luo and S. Deng, J. Colloid Interface Sci., 2013, 394, 445–450 CrossRef CAS PubMed.
- Y. Li, R. Cao, X. Wu, J. Huang, S. Deng and X. Lu, J. Colloid Interface Sci., 2013, 400, 78–87 CrossRef CAS PubMed.
- Y. Fu, Y. Zu, W. Liu, C. Hou, L. Chen, S. Li, X. Shi and M. Tong, J. Chromatogr. A, 2007, 1139, 206–213 CrossRef CAS PubMed.
- A. B. Pérez-Marín, V. M. Zapata, J. F. Ortuño, M. Aguilar, J. Sáez and M. Lloréns, J. Hazard. Mater., 2007, 139, 122–131 CrossRef PubMed.
- J. He, R. Lin, H. Long, Y. Liang and Y. Chen, J. Colloid Interface Sci., 2015, 454, 144–151 CrossRef CAS PubMed.
- Z. Aksu, Process Biochem., 2005, 40, 997–1026 CrossRef CAS.
- R. A. Chi, F. Zhou, K. Huang and Y. F. Zhang, Chin. J. Nat. Med., 2011, 9, 120–125 CAS.
- M. D. Saikia and N. N. Dutta, Colloids Surf., A, 2006, 280, 163–168 CrossRef CAS.
- J. Goscianska, M. Marciniak and R. Pietrzak, Chem. Eng. J., 2014, 247, 258–264 CrossRef CAS.
- S. Wang, Y. Boyjoo, A. Choueib and Z. H. Zhu, Water Res., 2005, 39, 129–138 CrossRef CAS PubMed.
- J. Dostál, J. Chem. Educ., 2000, 77, 993–998 CrossRef.
- C. Tien and B. V. Ramarao, Sep. Purif. Technol., 2014, 136, 303–308 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- G. S. Bohart and E. Q. Adams, J. Am. Chem. Soc., 1920, 42, 523–544 CrossRef.
- Y. H. Yoon and J. H. Nelson, Am. Ind. Hyg. Assoc. J., 1984, 45, 509–516 CrossRef CAS PubMed.
- H. C. Thomas, J. Am. Chem. Soc., 1944, 66, 1664–1666 CrossRef CAS.
- R. M. Clark, Environ. Sci. Technol., 1987, 21, 573–580 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1, Fig. S1 and S2. See DOI: 10.1039/c6ra01257d |
| This journal is © The Royal Society of Chemistry 2016 |