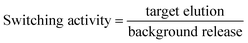Portable detection of ochratoxin A in red wine based on a structure-switching aptamer using a personal glucometer†
Chunmei Gua,
Feng Long*b,
Xiaohong Zhoua and
Hanchang Shi*a
aState Key Joint Laboratory of ESPC, School of Environment, Tsinghua University, Beijing 100084, China. E-mail: hanchang@tsinghua.edu.cn
bSchool of Environment and Natural Resources, Renmin University of China, Beijing, 100872, China. E-mail: longf04@mails.tsinghua.edu.cn
First published on 17th March 2016
Abstract
Ochratoxin A (OTA) detection is important for food safety and public health because OTA is one of the most toxic and widespread mycotoxins. In this study, a portable aptasensor was developed for OTA quantification using a structure-switching aptamer and a commercially available personal glucometer (PGM), which is an affordable portable device for home or field use. The OTA aptamer was directly immobilized onto magnetic beads (MBs) through a facile streptavidin–biotin reaction. Invertase-labeled competitor DNA that was partly complementary to the aptamer was initially hybridized with the aptamer to form a competitor DNA–aptamer–MB complex. The addition of OTA triggered the structure-switching of the aptamer from the aptamer–competitor DNA duplex to the aptamer–OTA complex, thus releasing the competitor–invertase. The released competitor–invertase subsequently catalyzed sucrose hydrolysis to glucose, which was measured using a PGM. A higher OTA concentration led to higher competitor–invertase release and higher glucose concentration. The length and concentration of the competitor DNA were optimized to obtain the highest structure-switching activity of the aptamer on the MBs. This activity is essential to improve the detection performance of OTA aptasensors. The optimal pH for sucrose hydrolysis to glucose was also determined. Under optimal conditions, the detection limits in buffer and red wine were of 3.31 and 3.66 μg L−1, respectively. This aptasensor also showed considerable selectivity to two other mycotoxins (aflatoxins B1 and B2). To the best of our knowledge, this paper was the first to report a portable aptasensor for OTA detection in real samples with the use of a PGM.
1. Introduction
Ochratoxin A (OTA) is a ubiquitous mycotoxin found in improperly stored food products.1 The immunotoxic, hepatotoxic, teratogenic, neurotoxic, and carcinogenic properties of OTA make it a potential human carcinogen (2B), as classified by the International Agency for Research on Cancer (IARC).2 Currently, OTA detection in food sources is usually performed using conventional chromatographic methods, such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), or gas chromatography (GC) coupled to UV, fluorescence, or mass spectrometry.3–8 Although these techniques are accurate with low detection limits (i.e., ng L−1), they require sophisticated and expensive instrumentation, careful calibration, and long analysis time, thereby limiting on-site, real-time, or portable detection. Several immunoassays have been developed for rapid OTA detection.5,9–13 However, antibody preparation is restricted because of the difficulty in growing certain hybridomas in vivo.14 In addition, antibody is a fragile, temperature-sensitive protein and may undergo irreversible denaturation.14 Aptamers are an emerging class of molecules that compete with antibodies in diagnostics14 and have been widely applied for biosensing and bioimaging because they can be chemically synthesized. Aptamers also have predictable and tailorable structures that permit facile conjugation to signal transduction agents, such as fluorophores or electrochemical agents.15–18 Numerous aptasensors have been developed for high-sensitive detection of small molecules, proteins, cells, and bacteria.19–22 Among them, structure-switching-based aptasensors have been applied to detect trace analytes by using a signaling aptamer, which can switch from DNA–DNA duplex to DNA–target when the targets are added to the detection system. Some aptamers have been obtained using the structure-switching SELEX method, which directly generates unmodified aptamers that can immediately be transformed into effective signaling probes without further optimization.23–25An OTA aptamer with high affinity and specificity was isolated using the conventional SELEX method in 2008.26 This aptamer showed some structure-switching activity.2,24,26–31 Several aptasensors for OTA detection based on the structure-switching of OTA aptamer have been reported. However, most of these methods were unsuitable for rapid OTA detection with an affordable device for home or field use. Moreover, although all of the aforementioned studies showed that the OTA aptamer has the inherent ability to switch its structure from DNA–DNA duplex to DNA–target in a heterogeneous phase [e.g., aptamer on magnetic beads (MBs) surface], only a few studies focused on the switching activity of aptamers on MBs. The competition between the aptamer–target and aptamer–competitor DNA duplex is a major consideration in designing a structure-switching aptasensor.28 Either too strong or inadequate binding of Watson–Crick DNA base pairing between the aptamer and competitor DNA may induce low target elution or high background release, thus decreasing the switching activity. However, a high switching activity of aptamers is essential to improve the detection performance of aptasensors. Hence, the competitor DNA should be carefully designed for a special aptamer. A suitable competitor DNA should possess sufficient hybridization ability with the aptamer to form a stable DNA–DNA duplex, and release of the competitor DNA upon addition of a target should be possible. The competitor length, DNA–DNA complex concentration on MBs, incubation pH, and incubation time are important factors in obtaining high switching activity of an aptamer on MBs.
Personal glucometers (PGMs) are widely used because they are readily available and affordable with the advantages of portability (i.e.,“pocket” size), reliable quantitative results, low cost, and easy-to-use. Researchers realized a portable and low-cost quantitative detection of many targets in addition to glucose by combining a PGM and the binding reaction of biomolecules (e.g., DNA and antibody).13,32–35 A novel structure-switching based aptasensor was developed in the present study for OTA quantification by using a commercially available PGM. The switching activity of OTA aptamer on MBs was studied to improve the OTA aptasensor performance. The length and concentration of competitor DNA, pH, and incubation time were optimized. The effect of pH on the catalytic performance of the invertase-labeled competitor DNA was also studied to shorten the detection time.32 A portable and quantitative OTA detection method in red wine matrix was realized for the first time based on the structure-switching aptamer using the PGM.
2. Experimental
2.1 Materials and apparatus
All DNA sequences were ordered from Sangong Biotech (Shanghai, China). Streptavidin magnetic beads were purchased from New England Biolabs Inc. (MA, USA). Invertase and sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC) were purchased from Sigma-Aldrich (Shanghai, China). Tris(2-carboxyethyl)phosphine hydrochloride (TCEP-HCl) was obtained from Pierce (Thermo Fisher, USA). General chemicals for buffer preparation were acquired from Alfa Aesar (MA, USA). OTA was purchased from Pribolab Pte. Ltd. (Singapore). Aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2) were obtained from Sigma-Aldrich (Shanghai, China). The ACCU-CHEK® Active Glucose Meter and the strips were from ROCHE (BASEL, Switzerland). Fluorescence spectrometer F-7000 was from Hitachi (Japan). The buffer components for OTA detection (1× buffer) were 10 mM Tris, 120 mM NaCl, 20 mM CaCl2, and 5 mM KCl, 0.01% Tween, pH 7.0. The DNA sequences used in this research are as follows (from left to right: 5′ to 3′):OTA aptamer: biotin-AAAAAAGATCGGGTGTGGGTGGCGTAAAGGGAGCATCGGACA
FAM-competitor-10 nt: FAM-AAAAAAAAAAAATGTCCGATGC
FAM-competitor-11 nt: FAM-AAAAAAAAAAAATGTCCGATGCT
FAM-competitor-12 nt: FAM-AAAAAAAAAAAATGTCCGATGCTC
FAM-competitor-13 nt: FAM-AAAAAAAAAAAATGTCCGATGCTCC
FAM-competitor-14 nt: FAM-AAAAAAAAAAAATGTCCGATGCTCCC
SH-competitor-10 nt: thiol-AAAAAAAAAAAATGTCCGATGC
SH-competitor-11 nt: thiol-AAAAAAAAAAAATGTCCGATGCT
SH-competitor-12 nt: thiol-AAAAAAAAAAAATGTCCGATGCTC
2.2 DNA–invertase conjugation
The conjugation procedure was similar to the previously reported method with some minor modifications.32,36 Purification was by molecular weight cut off filters (Amicon-3K and Amicon-100K). Briefly, 30 μL of 1 mM thiol–DNA, 2 μL of 1 M sodium phosphate buffer at pH 5.5, and 2 μL of 30 mM TCEP-HCl were mixed and kept at room temperature for 1 h. The excess TCEP was subsequently removed in Amicon-3K by using buffer A (0.1 M NaCl, 0.1 M sodium phosphate buffer, pH 7.3); this process was repeated for eight times. Meanwhile, 1 mg of sulfo-SMCC was mixed with 400 μL of 20 mg mL−1 invertase in buffer A without Tween-20. After vortexing, the solution was placed on a shaker for 1 h at room temperature. The mixture was centrifuged to remove excess insoluble sulfo-SMCC. The supernatant was purified in Amicon-100K by using buffer A without Tween-20; this process was repeated for eight times. The resulting solution of TCEP-activated thiol–DNA and sulfo-SMCC-activated invertase was mixed and kept at room temperature for 48 h. To remove unreacted thiol–DNA, the solution was purified in Amicon-100K by using Millipore water and then gradually changing to 1× buffer; this process was repeated for eight times.2.3 Switching activity assay of OTA aptamer on MBs
At room temperature, 10 pmol OTA aptamer and 12 pmol competitor DNA were mixed in 1× buffer (10 mM Tris, 120 mM NaCl, 20 mM CaCl2, and 5 mM KCl, 0.01% Tween, pH 7.0) for 30 min. The mixture was subsequently incubated with 20 μL of streptavidin MBs at room temperature for 30 min. After binding to the MBs, the competitor–aptamer–MBs complex was washed with 1× buffer for five times to remove the excess competitor–aptamers, which were non-specifically adsorbed onto the MBs. Thereafter, the complex was incubated in 40 μL of 1× buffer for 10 min, and the supernatant was taken as the background release. Subsequently, 40 μL of OTA was added to the competitor–aptamer–MBs and incubated for another 10 min. The OTA-eluted supernatant was taken as target elution. The switching activity was calculated as242.4 Effect of pH on invertase catalytic performance
100 μg mL−1 invertase at different pH values (2.96, 3.95, 4.44, 5.43, 6.34, 7.3, and 8.5) were prepared using Na2HPO4–citric acid buffer. To 15 μL of 100 μg mL−1 invertase solution mentioned above, 5 μL of 2 M sucrose was added and allowed to react at room temperature for 5 min. The mixture was tested using a PGM, and the glucose concentration readout was recorded.2.5 OTA detection procedures using a PGM-based aptasensor
In 50 μL of 1× buffer, 500 pmol OTA aptamer and 600 pmol competitor–invertase were initially mixed at room temperature for 30 min. Subsequently, 240 μL of streptavidin-coated MBs, which had been buffer-exchanged to 1× buffer at room temperature for 30 min, were added to the mixture and reacted at room temperature for 30 min. After binding to the MBs, the mixture was washed with 1× buffer for five times and 20 μL portions of MBs were distributed to each Eppendorf tube. Subsequently, 40 μL of OTA at various concentrations in 1× buffer or in 1× buffer with different red wine concentrations were added into each Eppendorf tube, and were shaken for 15 min at room temperature. After mixing on the magnetic strand, 15 μL of the supernatant was taken and added to 5 μL of 2 M sucrose. After reaction at room temperature for 30 min, the glucose content was measured by using a PGM.3. Results and discussion
3.1 Switching activity assay of OTA aptamer on MBs
Instead of using the indirect 3-strand DNA strategy in which an extra BDNA was needed to enable the immobilization of DNA aptamer onto MBs, in this paper, we directly immobilize OTA aptamer onto MBs.25 To obtain the highest switching activity of the aptamer on MBs, the competitor DNA should have an appropriate length that could be hybridized with the aptamer and subsequently be eluted by OTA addition. Competitors of varying lengths (10 nt, 11 nt, 12 nt, 13 nt, and 14 nt) were synthesized (Fig. 1a).23 To verify the binding capacity of the different competitor DNA length with OTA aptamer, FAM-competitor DNA (10 nt, 12 nt, 13 nt, and 14 nt) were used to quantify the amount of the competitor–aptamer complex on MBs. Fig. S1† shows that the fluorescence signal of the mixture of FAM-competitor DNA and biotinylated OTA aptamer decreased after the FAM-competitor–aptamer complex was separated by the streptavidin MBs. This result demonstrated that the FAM-competitor successfully hybridized with the OTA aptamer. When the ratio of the added aptamer and competitor was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, the total efficiency of DNA hybridization and biotin–streptavidin coupling was estimated at 70%. To verify whether the structure-switching aptasensor on MBs worked, two concentrations of OTA (1 mg L−1 and 10 μg L−1) were applied to test the switching activity. The result showed that at 1 mg L−1 OTA, 10 nt, 11 nt, 12 nt, 13 nt, and 14 nt competitor molecules showed switching activity of 8.5, 10.9, 10.8, 3.1, and 1.7, respectively. With the increase in competitor length, the background release decreased and the amount of competitor elution induced by target was lowered because of the stronger competitor–OTA aptamer binding force (Fig. S2†). Competitors 11 nt and 12 nt showed the highest switching activity (Fig. 1b). At high OTA elution concentration, competitors 10 nt, 11 nt, and 12 nt were suitable candidates for the sensor design. Given that OTA is a biotoxin in food or other matrix and a small amount of OTA may cause damage to human bodies, detection of low concentrations of OTA at the 3–10 ng g−1 safety level in food is an urgent task.12 Therefore, the effect of low OTA concentration (10 μg L−1) on the switching activity was further studied. As shown in Fig. 1b and S3,† at 10 μg L−1 OTA, only competitors 10 nt and 11 nt showed switching activity above 1 (i.e., 1.5). For competitors 12 nt, 13 nt, and 14 nt, the aptamer–competitor DNA duplex assembly was too strong, and 10 μg L−1 OTA could not induce structure switching; while the duplex assembly of aptamer–competitor-10 nt and 11 nt allowed OTA to gradually replace them in the assembly.37 To obtain a broad dynamic range and lower background release, competitor 11 nt was selected for the further switching activity assay. It should also be pointed that direct immobilization of OTA aptamer onto MBs didn't cause the loss of switching activity.
1.2, the total efficiency of DNA hybridization and biotin–streptavidin coupling was estimated at 70%. To verify whether the structure-switching aptasensor on MBs worked, two concentrations of OTA (1 mg L−1 and 10 μg L−1) were applied to test the switching activity. The result showed that at 1 mg L−1 OTA, 10 nt, 11 nt, 12 nt, 13 nt, and 14 nt competitor molecules showed switching activity of 8.5, 10.9, 10.8, 3.1, and 1.7, respectively. With the increase in competitor length, the background release decreased and the amount of competitor elution induced by target was lowered because of the stronger competitor–OTA aptamer binding force (Fig. S2†). Competitors 11 nt and 12 nt showed the highest switching activity (Fig. 1b). At high OTA elution concentration, competitors 10 nt, 11 nt, and 12 nt were suitable candidates for the sensor design. Given that OTA is a biotoxin in food or other matrix and a small amount of OTA may cause damage to human bodies, detection of low concentrations of OTA at the 3–10 ng g−1 safety level in food is an urgent task.12 Therefore, the effect of low OTA concentration (10 μg L−1) on the switching activity was further studied. As shown in Fig. 1b and S3,† at 10 μg L−1 OTA, only competitors 10 nt and 11 nt showed switching activity above 1 (i.e., 1.5). For competitors 12 nt, 13 nt, and 14 nt, the aptamer–competitor DNA duplex assembly was too strong, and 10 μg L−1 OTA could not induce structure switching; while the duplex assembly of aptamer–competitor-10 nt and 11 nt allowed OTA to gradually replace them in the assembly.37 To obtain a broad dynamic range and lower background release, competitor 11 nt was selected for the further switching activity assay. It should also be pointed that direct immobilization of OTA aptamer onto MBs didn't cause the loss of switching activity.
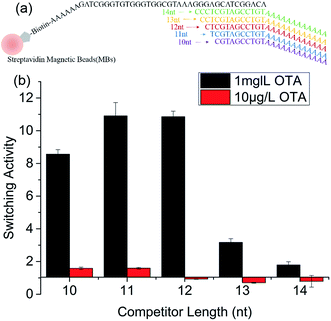 | ||
| Fig. 1 Optimization of the length of competitor DNAs. (a) Competitor DNAs were used in the aptasensor; (b) Effect of the DNA competitor length on switching activity of OTA aptamer on MBs. | ||
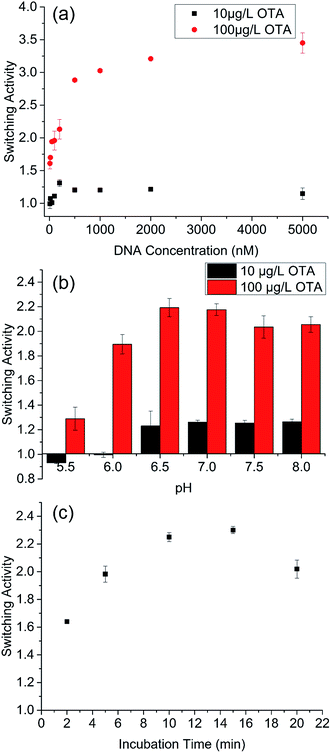
Invertase concentration is a critical factor in developing a PGM-based sensor because it affects detection time. At low invertase concentration, the catalytic reaction from sucrose to glucose would take a long time. To shorten the detection period, high concentration of invertase should be used in a PGM-based sensor. However, the invertase in the current PGM-based sensor existed in the form of DNA–invertase conjugates. To increase invertase concentration, the DNA–invertase concentration should be increased in the detection process. Subsequently, switching activity at different DNA concentrations was further studied. The results are shown in Fig. 2a. At 100 μg L−1 OTA, the switching activity apparently increased with increasing DNA concentration, particularly when the concentration increased from 10 nM to 500 nM. At 10 μg L−1 OTA, the switching activity did not considerably change with DNA concentration from 10 nM to 100 nM (range of 1–1.1). However, the switching activity peaked at 200 nM DNA with a value of 1.3, and consequently ranged between 1.2 and 1.3 with increasing DNA concentration.
Switching activity of the aptamer on MBs at different pH levels was also tested and the result is shown in Fig. 2b. A DNA concentration of 200 nM was used in this set of experiment. No switching activity was detected when pH was less than 5.0, either with 10 or 100 μg L−1 OTA, because few DNA molecules (both background release and target elution) could be released from the aptamer–competitor–MBs (Fig. S4†). With 100 μg L−1 OTA elution, switching activity gradually increased with the increase in pH and reached the plateau at pH 6.5 (Fig. 2b). From pH 6.5 to 8.0, the switching activity became stable with small fluctuations. With 10 μg L−1 OTA elution, switching activity could only be observed at pH > 6 and remained stable from 6.5 to 8.0. Therefore, pH > 6 was suitable in the sensor development.
The switching activity kinetics from aptamer–competitor-11 nt to aptamer–target was further studied. As shown in Fig. 2c, the switching activity gradually increased from 2 min to 15 min incubation and then decreased to 20 min incubation. This change in switching activity maybe attributed primarily to the slower target elution of FAM-labeled competitor than that of the background release at 20 min incubation time (Fig. S5†). Therefore, 15 min incubation was used for the succeeding experiments. Compared with the kinetics in homogeneous phase (30 s to the equilibrium), longer time to reach equilibrium was observed on the MBs surface.29
3.2 Effect of pH on the catalytic performance of invertase
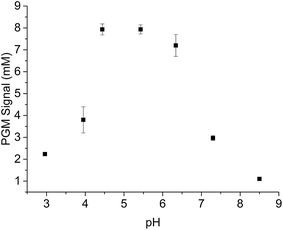
Invertase has properties that are similar to other enzymes, such as pH dependence. Optimal pH is responsible for catalytic hydrolysis of sucrose to glucose. Therefore, pH dependence of the invertase catalytic performance was tested. The highest catalytic performance was reached at around pH 4.5 and decreased gradually as the pH increased to 8.5 (Fig. 3). Given that a rapid catalytic process would decrease the detection period and the highest aptamer switching activity was observed at pH 6.5, pH 6.5 was applied in the current PGM-based sensor.3.3 OTA detection mechanism based on the switching activity of aptamer on MBs with the use of a portable PGM
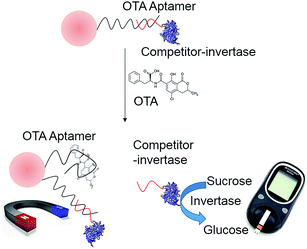
Fig. 4 illustrates the design of a portable structure-switching aptasensor to detect OTA using a PGM. A competitor DNA–invertase conjugate was formed. The structure-switching OTA aptamer was immobilized onto the MBs and hybridized with the invertase-labeled competitor DNA. OTA addition triggered the structure switching from aptamer–competitor DNA duplex to aptamer–OTA, thus releasing the invertase-labeled competitor. Thereafter, the released competitor–invertase catalyzed sucrose hydrolysis to glucose, which could then be quantified using a PGM.3.4 OTA dose–response curve using a PGM
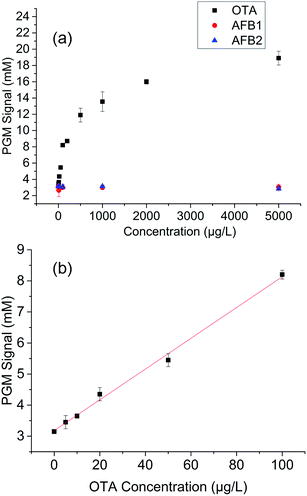
Competitor DNA–invertase conjugates with different lengths (10 nt, 11 nt, and 12 nt) were employed in the PGM sensor development considering that conjugation of invertase into the competitor–DNA might affect the switching activity. The results showed that competitor-11 nt–invertase still showed the highest switching activity (Fig. S6†). However, smaller standard curve slope can be obtained with competitor-11 nt–invertase than competitor-10 nt–invertase. Therefore, competitor-10 nt was chosen as the competitor DNA in the portable sensor development.Standard OTA solutions in the concentration range of 0–5000 μg L−1 were tested. As shown in Fig. 5a, the glucose signal linearly increased with increasing OTA concentration when OTA concentration was less than 100 μg L−1. A detection limit of 3.31 μg L−1 was calculated according to the definition of 3σ/slope (Fig. 5b). This sensitivity is better than that of previously reported PGM sensor based on the OTA antibody–antigen interaction (6.8 μg L−1)12 and the catalytic recycling fluorescent aptasensor (8.08 μg L−1).38 Moreover, the simple and easy-to-use PGM-based aptasensor in the current study can be conveniently used to detect OTA for home or field use because it only requires a readily available PGM instead of other sophisticated instruments.39–43 Furthermore, the adoption of aptamer instead of an antibody realized a more robust sensor with regard to production, storage, and stability. As shown in Fig. 5a, considerable selectivity of this aptasensor was also achieved because the glucose signal remained stable with increasing concentration of two other mycotoxins, namely, AFB1 and AFB2.
3.5 OTA detection in red wine matrix using a portable PGM-based aptasensor
To verify the applicability of the proposed portable sensor in real samples, various concentrations of red wine were added into the OTA buffer for detection. Switching activity of competitor DNA–aptamer–MBs induced by 10 and 100 μg L−1 OTA was initially tested using red wine at different concentrations, ranging from 2% to 20% (v/v). As shown in Fig. 6a, switching activity decreased gradually with the increase in red wine concentration. When the red wine concentration is 2% (v/v), the switching activity is 90% higher than that of the OTA buffer. The switching activity decreased to 65% when the red wine concentration further reached 5%. When the red wine concentration was higher than 10%, the switching activity was almost undetected. This result proved that high red wine concentration apparently inhibited the switching activity of the OTA aptamer on MBs. Therefore, 2% red wine was chosen for the actual sample testing of the PGM-based sensor. It should be noted that in our preliminary experiment, we found that no glucometer readout of the red wine background can be observed. Therefore, the carryover of sucrose or glucose by red wine was negligible in our real sample testing experiment. In addition, influence of contents in red wine on the activity of invertase was tested. As shown in Fig. S7,† when the red wine concentration (v/v) ranged from 0–5%, the activity of invertase almost kept stable.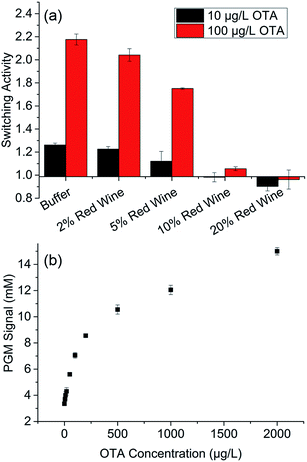 | ||
| Fig. 6 (a) Switching activity of OTA aptamer on MBs in buffer with red wine. (b) Performance of the portable sensor in buffer with 2% red wine. | ||
A titration curve of the samples containing 2% red wine with increasing OTA amount (0–2000 μg L−1) is shown in Fig. 6b, in which a corresponding increase in glucose concentration was detected by the PGM. A detection limit of 3.66 μg L−1 was calculated according to the definition of 3σ/slope in 2% red wine. This detection limit was slightly higher than that of the buffer, which may be attributed to the effect of the red wine matrix. These results demonstrated that the developed method could be applied for OTA detection in real samples.
4. Conclusions
In this study, we developed a simple, portable, and low-cost structure-switching aptasensor to quantify OTA based on the competitor DNA–OTA aptamer–MBs complex sensing design, instead of the three-strand-DNA complex on MBs strategy.32 Direct immobilization of OTA aptamer onto the MBs did not cause the loss of switching activity. The length and concentration of competitor DNA were optimized to obtain the highest switching activity of the aptamer on the MBs, and the highest switching activity was obtained with the use of competitor-11 nt–OTA aptamer–MBs. The optimal pH was also determined for the catalytic reaction from sucrose to glucose. Switching activity kinetics of the competitor DNA–OTA aptamer–MBs demonstrated that the OTA induced structure-switching required only 15 min to reach equilibrium. Under optimal conditions, detection limits of 3.31 and 3.66 μg L−1 in buffer and 2% red wine, respectively, were obtained for OTA detection using the portable PGM-based aptasensor. The successful OTA detection in red wine demonstrated the feasibility of the developed portable aptasensor for analysis of real samples. Our sensing strategy could be readily extended to the simple and low-cost monitoring of other targets in real food or environmental samples with the use of other functional aptamers.Acknowledgements
This work was supported by the National Instrument Major Project of China (2012YQ030111).References
- A. Pfohl-Leszkowicz and R. A. Manderville, Mol. Nutr. Food Res., 2007, 51, 61–99 CAS.
- J. Chen, X. Zhang, S. Cai, D. Wu, M. Chen, S. Wang and J. Zhang, Biosens. Bioelectron., 2014, 57, 226–231 CrossRef CAS PubMed.
- P. Zöllner, A. Leitner, D. Lubda, K. Cabrera and W. Lidner, Chromatographia, 2000, 52, 818–820 Search PubMed.
- R. Ghali, K. Hmaissia-khlifa, H. Ghorbel, K. Maaroufi and A. Hedili, Food Control, 2009, 20, 716–720 CrossRef CAS.
- D. Flajs, A. M. Domijan, D. Ivić, B. Cvjetković and M. Peraica, Food Control, 2009, 20, 590–592 CrossRef CAS.
- I. Y. Goryacheva, S. De Saeger, M. Lobeau, S. A. Eremin, I. Barna-Vetró and C. Van Peteghem, Anal. Chim. Acta, 2006, 577, 38–45 CrossRef CAS PubMed.
- P. Songsermsakul and E. Razzazi-Fazeli, J. Liq. Chromatogr. Relat. Technol., 2008, 31, 1641–1686 CrossRef CAS.
- N. W. Turner, S. Subrahmanyam and S. A. Piletsky, Anal. Chim. Acta, 2009, 632, 168–180 CrossRef CAS PubMed.
- X.-H. Wang, T. Liu, N. Xu, Y. Zhang and S. Wang, Anal. Bioanal. Chem., 2007, 389, 903–911 CrossRef CAS PubMed.
- I. Barna-Vetró, L. Solti, J. Téren, Á. Gyöngyösi, E. Szabó and A. Wölfling, J. Agric. Food Chem., 1996, 44, 4071–4074 CrossRef.
- K. Thirumala-Devi, M. Mayo, G. Reddy, S. Reddy, P. Delfosse and D. Reddy, J. Agric. Food Chem., 2000, 48, 5079–5082 CrossRef CAS PubMed.
- Y. Xiang and Y. Lu, Anal. Chem., 2012, 84, 4174–4178 CrossRef CAS PubMed.
- D. Tang, Y. Lin, Q. Zhou, Y. Lin, P. Li, R. Niessner and D. Knopp, Anal. Chem., 2014, 86, 11451–11458 CrossRef CAS PubMed.
- S. D. Jayasena, Clin. Chem., 1999, 45, 1628–1650 CAS.
- D. L. Robertson and G. F. Joyce, Nature, 1990, 344, 467–468 CrossRef CAS PubMed.
- C. Tuerk and L. Gold, Science, 1990, 249, 505–510 CAS.
- J. Liu, Z. Cao and Y. Lu, Chem. Rev., 2009, 109, 1948–1998 CrossRef CAS PubMed.
- X. Fang and W. Tan, Acc. Chem. Res., 2010, 43, 48–57 CrossRef CAS PubMed.
- J. Liu and Y. Lu, Nat. Protoc., 2006, 1, 246–252 CrossRef CAS PubMed.
- W. Xu and Y. Lu, Anal. Chem., 2010, 82, 574–578 CrossRef CAS PubMed.
- H.-M. So, D.-W. Park, E.-K. Jeon, Y.-H. Kim, B. S. Kim, C.-K. Lee, S. Y. Choi, S. C. Kim, H. Chang and J.-O. Lee, Small, 2008, 4, 197–201 CrossRef CAS PubMed.
- Y. Wang, Z. Li, D. Hu, C.-T. Lin, J. Li and Y. Lin, J. Am. Chem. Soc., 2010, 132, 9274–9276 CrossRef CAS PubMed.
- R. Nutiu and Y. F. Li, J. Am. Chem. Soc., 2003, 125, 4771–4778 CrossRef CAS PubMed.
- R. Nutiu and Y. F. Li, Angew. Chem., Int. Ed., 2005, 44, 1061–1065 CrossRef CAS PubMed.
- C. Gu, T. Lan, H. Shi and Y. Lu, Anal. Chem., 2015, 87, 7676–7682 CrossRef CAS PubMed.
- J. A. Cruz-Aguado and G. Penner, J. Agric. Food Chem., 2008, 56, 10456–10461 CrossRef CAS PubMed.
- A. D. Ellington and J. W. Szostak, Nature, 1992, 355, 850–852 CrossRef CAS PubMed.
- J. H. Chen, Z. Y. Fang, J. Liu and L. W. Zeng, Food Control, 2012, 25, 555–560 CrossRef CAS.
- J. A. Cruz-Aguado and G. Penner, Anal. Chem., 2008, 80, 8853–8855 CrossRef CAS PubMed.
- R. Wang, Y. Xiang, X. Zhou, L.-H. Liu and H. Shi, Biosens. Bioelectron., 2015, 66, 11–18 CrossRef CAS PubMed.
- H. Kuang, W. Chen, D. Xu, L. Xu, Y. Zhu, L. Liu, H. Chu, C. Peng, C. Xu and S. Zhu, Biosens. Bioelectron., 2010, 26, 710–716 CrossRef CAS PubMed.
- Y. Xiang and Y. Lu, Nat. Chem., 2011, 3, 697–703 CrossRef CAS PubMed.
- L. Yan, Z. Zhu, Y. Zou, Y. Huang, D. Liu, S. Jia, D. Xu, M. Wu, Y. Zhou, S. Zhou and C. J. Yang, J. Am. Chem. Soc., 2013, 135, 3748–3751 CrossRef CAS PubMed.
- Q. Wang, F. Liu, X. Yang, K. Wang, H. Wang and X. Deng, Biosens. Bioelectron., 2015, 64, 161–164 CrossRef CAS PubMed.
- Y. Zhao, D. Du and Y. Lin, Biosens. Bioelectron., 2015, 72, 348–354 CrossRef CAS PubMed.
- Y. Xiang and Y. Lu, Anal. Chem., 2012, 84, 1975–1980 CrossRef CAS PubMed.
- R. Nutiu and Y. Li, Chem.–Eur. J., 2004, 10, 1868–1876 CrossRef CAS PubMed.
- Y. Wei, J. Zhang, X. Wang and Y. Duan, Biosens. Bioelectron., 2015, 65, 16–22 CrossRef CAS PubMed.
- S. Xie, Y. Chai, Y. Yuan, L. Bai and R. Yuan, Biosens. Bioelectron., 2014, 55, 324–329 CrossRef CAS PubMed.
- P. Tong, W.-W. Zhao, L. Zhang, J.-J. Xu and H.-Y. Chen, Biosens. Bioelectron., 2012, 33, 146–151 CrossRef CAS PubMed.
- J. Zhang, X. Zhang, G. Yang, J. Chen and S. Wang, Biosens. Bioelectron., 2013, 41, 704–709 CrossRef CAS PubMed.
- L. Yang, Y. Zhang, R. Li, C. Lin, L. Guo, B. Qiu, Z. Lin and G. Chen, Biosens. Bioelectron., 2015, 70, 268–274 CrossRef CAS PubMed.
- L. Huang, J. Wu, L. Zheng, H. Qian, F. Xue, Y. Wu, D. Pan, S. B. Adeloju and W. Chen, Anal. Chem., 2013, 85, 10842–10849 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27880e |
| This journal is © The Royal Society of Chemistry 2016 |