Optical sensitivity of mussel protein-coated double-walled carbon nanotubes on the iron–DOPA conjugation bond†
Abstract
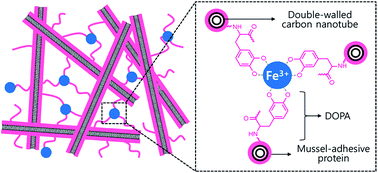
The optical properties of semiconducting carbon nanotubes respond sensitively to external conditions including the formation of chemical bonds. In order to detect the iron–3,4-dihydroxy-L-phenylalanine (DOPA) conjugation bonds with metal ions, an individually dispersed double-walled carbon nanotube (DWNT) suspension was prepared via homogeneous coating of mussel adhesive protein (MAP). MAP exhibited a high ability for individually dispersing the bundled DWNTs through strong physical interactions with the outer tubes. We demonstrated sensitively altered optical properties of the DWNT suspension upon addition of FeCl3 solution via the formation of coordinative bonds between DOPA in MAP and Fe3+ ions. The iron–DOPA bonds acted as electron acceptors and thus provided a favourable non-radiative channel for the optical depression of signal from semiconducting inner tubes in DWNT suspension. Several physical and chemical effects on the sensitively quenched photoluminescence of semiconducting inner tubes were explained based on the iron–DOPA bonds. We also observed that the DOPA groups in MAP were fully saturated at ca. 376.7 mol% of Fe3+ ions for MAP.

 Please wait while we load your content...
Please wait while we load your content...