An assessment of the potential of invasive weeds as multiple feedstocks for biofuel production†
Arup Jyoti Boraha,
Shuchi Singha,
Arun Goyalab and
Vijayanand S. Moholkar*ac
aCenter for Energy, Indian Institute of Technology Guwahati, Guwahati-781 039, Assam, India. E-mail: vmoholkar@iitg.ernet.in; Fax: +91-361-258-2291; Tel: +91-361-258-2251
bDepartment of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati-781 039, Assam, India
cDepartment of Chemical Engineering, Indian Institute of Technology Guwahati, Guwahati-781 039, Assam, India
First published on 26th April 2016
Abstract
The present study assessed the feasibility of five invasive weeds, namely, Arundo donax, Saccharum spontaneum, Mikania mikrantha, Lantana camara and Eichhornia crasspies, as a feedstock for biofuels production. The yield of total fermentable sugars from the pretreatment and enzymatic hydrolysis of these biomasses was assessed. However, the pretreatment and enzymatic hydrolysis were carried out at conditions optimized for the biomass of P. hysterophorus and thus, the conditions of pretreatment/enzymatic hydrolysis were not specifically optimized for any of the invasive weeds. Despite this, it was revealed that the average yield of total fermentable (hexose + pentose) sugars from all the weeds was 43.85 g per 100 g of raw biomass, which corresponds to a theoretical yield of 27.36 g ethanol and 17.96 g butanol. These yields are comparable to bioalcohol yields from the biomass of P. hysterophorus under optimized pretreatment conditions. Characterization of the biomass was carried out using X-ray diffraction, FTIR and SEM micrographs. The high yields of fermentable sugars obtained herein from invasive weeds, even under un-optimized pretreatment conditions, clearly point towards the feasibility of biorefinery using these weeds as multiple feedstocks for the production of alcoholic biofuels.
1. Introduction
The quest for alternative and renewable liquid transportation fuel has intensified over the past few years due to the fast and continuing depletion of fossil fuels threatening energy security, and due to increasing concerns about climatic change due to the large emission of greenhouse gases through vehicular exhausts. Waste biomasses in the form of noxious invasive weeds (in addition to conventional lignocellulosic substrates of agro- and forestry residues)1,2 could form a large potential feedstock source for economic biofuel production.3,4 Large numbers of such invasive species exist in India. These species infest millions of hectares of arable and non-arable (or infertile) land, leading to enormous monetary loss due to a reduction in crops and forage production. The actual biomass produced by these noxious weeds is in the range of 15–20 tons per hectare. Nonetheless, these biomasses can form a feedstock for liquid biofuels due to their significant content of holocellulose (in addition to lignin), which can be hydrolyzed to produce fermentable monomeric sugars. Hexose and pentose sugars produced from the hydrolysis of cellulose and hemicellulose can be used to produce alcoholic biofuels, such as ethanol and butanol.The pretreatment of biomass prior to fermentation encompasses the removal of lignin and the acid/enzymatic hydrolysis of hemicellulose and cellulose fractions to pentose and hexose sugars. Removal of the lignin matrix in biomass causes a better exposure of the cellulose and hemicellulose fraction to acid/enzymatic action, which leads to enhancement of the sugar yield. The pretreatment of biomass is also aimed at a reduction in the crystallinity of cellulose and in increasing the biomass porosity and surface area, which all contribute to a faster and higher hydrolysis of the cellulose/hemicellulose, thus maximizing the yield of fermentable sugars.
1.1 Aim and approach
The use of a cheap feedstock and optimization of the cost-intensive pretreatment techniques are crucial aspects of the economic feasibility of a sustainable biofuels process. In the present study, we dealt with the pretreatment of multiple invasive species or weeds (which are essentially waste biomasses), and determined the yield of total (hexose + pentose) reducing (or fermentable) sugars, which is a measure of their potential as a feedstock for biofuels. The invasive weeds considered in this work are: (1) Arundo donax (AD), (2) Saccharum spontaneum (SS), (3) Mikania micrantha (MM), (4) Lantana camara (LC) and (5) Eichhornia crassipes (EC). A number of previous authors have also addressed the matter of the pretreatment of these waste biomasses and a summary of some representative studies in optimization of the pretreatment and fermentation of these invasive weeds is given in Table 1. Most of these studies include the following components: acid hydrolysis, delignification, enzymatic hydrolysis and fermentation of the acid/enzyme hydrolyzates to bioethanol. It could be inferred from Table 1 that the optimum pretreatment conditions differ significantly for each biomass.| Reference | Biomass | Type and details of pretreatment | Major observations and conclusions |
|---|---|---|---|
| Komolwanich et al.5 | Arundo donax | Microwave assisted alkali pretreatment | 120 °C/5 min/5% w/v NaOH. Total sugar release: 6.8/100 g biomass |
| Two stages pretreatment microwave/dilute NaOH followed by microwave/dil. H2SO4 | 120 °C/5 min/5% w/v NaOH and 180 °C/30 min/0.5% w/v H2SO4 31.99/100 g biomass | ||
| Glucose is the main monomeric sugars | |||
| Scordia et al.6 | Arundo donax | Optimization of enzymatic hydrolysis (EH) and simultaneous saccharification and fermentation (SSF). Optimization of EH and SSF using response surface methodology (RSM) with 2 input parameters: severity factor (SF) and oxalic acid concentration (OA) | Xylan content after dil. oxalic acid pretreatment reduced with increasing SF and OA. Glucan and lignin showed opposite trend with respect to xylan content after dil. OA treatment |
| Temp. range: 150–190 °C | Final results: glucan conversion in EH = 95%, and ethanol production = 18 g L−1 (75% of maximum theoretical yield) for pretreatment conditions of SF = 4.05 and OA = 5% w/w | ||
| Treatment time range: 10–40 min | |||
| Dil. oxalic acid concentration range = (2–8% w/v) | |||
| You et al.7 | Arundo donax | Cost effective pretreatment of biomass with protic acid resin Amberlyst 35DRY and ionic liquid 1-butyl-3-methylimidazolium chloride | Reduction in cellulose crystallinity and increased porosity due to extensive swelling of undissolved biomass and partial depolymerization of longer cellulose chains of dissolved biomass by Amberlyst catalyst |
| Two stage treatment: (1) 160 °C with ionic liquid for 1.5 h, (2) slurry of ionic liquid and Amberlyst 35DRY resin at 90 °C for 1 h | High glucose yield of 92.8% than 42.8% yield for single ionic liquid treatment for enzyme loading of 20 FPU per g of substrate. Reusability of the ionic liquid solid catalyst system | ||
| Anderson et al.8 | Arundo donax | 1.75% w/v H2SO4 (8.5 mL), 121 °C h−1 | Xylan associated monosaccharide yield: 201 mg g−1 |
| 1.2 mL of 10% w/v Ca(OH)2 | Ethanol produced from A. donax: 109 mg g−1 | ||
| GC 220 cellulase 5FPU per g biomass | |||
| 12U Novozyme 188 cellubiase | |||
| Scordia et al.9 | Arundo donax | Dilute acid pretreatment for following conditions: | S. carlsbergensis results: max ethanol concentration of 15.9 g L−1 after 48 h at pH 5 |
| Effect of temperature (170 °C–190 °C) | S. stipitis results: ethanol concentration of 15.9 g L−1 in 96 h. Increasing pH to 6 reduced lag phase and attained 18 g L−1 after 72 h | ||
| Acid loading (2–10% w/v); reaction time (15–40 min) | |||
| Simultaneous saccharification and fermentation using commercial enzymes and two yeast strains: S. stipitis CBS 6054 and S. carlsbergensis FPL-450 | |||
| Kuila et al.10 | Lantana camara | Laccase treatment followed by simultaneous saccharification and fermentation (SSF); crude laccase 400 IU per mL, pH 6.5; 10 g dry substrate liq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solid (2 mL g−1) kept 8 h at 37 °C solid (2 mL g−1) kept 8 h at 37 °C |
Maximum bioethanol production: 5.14% v/v |
| SSF using cellulase from T. reesei. Optimization of SSF through CCD based response surface methodology | Optimum substrate concentration: 17% w/v | ||
| Optimum inoculum volume: 9% v/v | |||
| Inoculum age 60 h and 144 h of incubation time | |||
| Enhanced bioethanol concentration of 6.01% v/v using mutant strain of S. cerevisiae | |||
| Gupta et al.11 | Lantana camara | Biomass pretreatment with acid, alkali and chlorite to improve enzymatic saccharification of cellulose | Chlorite treatment removes maximum lignin with 90% w/w residual holocellulose |
| (1) For 100 g substrate: acid concentration 1–5% w/v, time 15–16 min, temperature 121 °C | Results of enzymatic hydrolysis: | ||
| (2) For 100 g substrate: alkali concentration 1–5% w/v for 2 h. Thermal pretreatment at 121 °C; time 15, 30, 45 and 60 min | (1) Chlorite treatment: 90% w/w initial holocellulose, saccharification of 86–92% fraction | ||
| (3) Chlorite pretreatment: sodium chlorite 1–5% w/v for 121 °C for 15, 30, 45, 60 min followed by washing and drying at 60 °C | (2) Alkali treatment: 66–76% w/w initial holocellulose, saccharification of 55% fraction | ||
| (3) Acid treatment: 39.5–48% w/w initial holocellulose, saccharification of 38–48% fraction | |||
| Kuhad et al.12 | Lantana camara | Dilute acid treatment at 3% w/v H2SO4 at 120 °C/45 min followed by delignification using sodium sulphite (5% w/v) and sodium chlorite (3% w/v) | Maximum sugar yield with dil. acid hydrolysis: 187.14 mg g−1 total sugar with inhibitors such as phenolics, furfurals and hydroxyl methyl furfurals |
| Detoxification of acid hydrolyzate using overliming and activated charcoal | 87.2% lignin removal with sodium sulphate and sodium chlorite treatment | ||
| Enzymatic hydrolysis of pretreated and delignified biomass; fermentation of acid and enzymatic hydrolyzate with P. stipitis and S. cerevisiae | Fermentation of acid and enzyme hydrolyzate with P. stipitis and S. cerevisiae yielded 5.16 g L−1 (yield 0.32 g g−1) and 17.7 g L−1 (yield 0.48 g g−1) ethanol in 24 and 16 h, respectively | ||
| Kataria et al.13 | Saccharum spontaneum | Biomass loading 5% (w/v); alkaline (NaOH) pretreatment at different concentrations (0.5, 1, 1.5, 2% w/v); period of treatment: 30, 60, 90, 120 min at 121 °C. Enzymatic hydrolysis of biomass with crude mixture of cellulase enzymes (20 units per g biomass); CMCase, FPAse, xylanase activities of 1.41, 1.12 and 6.23 units, respectively | Optimization results: (1) 70.75% lignin removal for 0.5% w/v NaOH treatment at 120 °C with total reducing sugar yield in enzymatic saccharification: 350 mg g−1 |
| (2) Holocellulose increase in biomass from 64.7% to 79.61% | |||
| (3) 79.3% lignin removal for 2% w/v NaOH in 90 min treatment. Holocellulose content increases to 76.7% with 70 mg g−1 reducing sugar yield in enzymatic saccharification | |||
| Chaudhury et al.14 | Saccharum spontaneum | Different alkaline pretreatment methods (NaOH, NaOH + 10% urea and aqueous ammonia) were optimized for maximum delignification. Solubilization of solid residue using H2SO4 60% (v/v), 10% biomass loading at 30 °C for 4 h | Maximum delignification with alkaline treatment: (1) 47.8% from 7% NaOH, 48 h, and 10% biomass loading; (2) 51% from NaOH + urea (7% NaOH + 10% urea, 48 h and 10% biomass loading); (3) 48% from 30% ammonia (40 days and 10% biomass loading) |
| Real hydrolysis of cellulose and hemicellulose with diluted slurry with acid concentration of 10% at 100 °C for 1 h | |||
| Best result for reducing sugar yield with ammonia treated biomass: 0.58 g reducing sugar per g of initial biomass after acid hydrolysis. This accounts for nearly 85% of the total sugars present in the biomass | |||
| Chandel et al.15 | Saccharum spontaneum | Three different pretreatment approaches viz. dil. sulfuric acid (1.5% v/v at160 °C), dil. sodium hydroxide (0.4% w/v or 0.1 N at 120 °C), and aq. ammonia (15%) treatment at 50 °C and 24 h followed by enzymatic hydrolysis (5–35 FPU per g of dry substrate) | A max. sugar yield of 631.5 ± 3.25 mg g−1 with 89.38% hydrolytic efficiency (HE) after enzymatic hydrolysis of aq. ammonia pretreated biomass |
| Fermentation results: yields of 0.36 g g−1 from acid hydrolyzate, 0.384 g g−1 from enzymatic hydrolyzate of acid pretreated substrate, 0.391 g g−1 from enzymatic hydrolyzate of alkali pretreated substrate and 0.4 g g−1 from enzymatic hydrolyzate of aq. ammonia pretreated substrate | |||
| Yan et al.16 | Eichhornia crassipes (water hyacinth) | NaOH/H2O2-pretreated water hyacinth | Reducing sugar yield of 223.53 mg g−1 dry biomass with reduced cellulose crystallinity |
| 1.5% v/v H2O2 and 3% (w/v) NaOH at 25 °C | |||
| Phothisantikul et al.17 | Eichhornia crassipes (water hyacinth) | Temperature range 160–220 °C, hydrothermal pretreatment using ball-mill reactor followed by enzymatic hydrolysis | Glucose yield at 220 °C in absence of CH3COOH and K2CO3 = 0.267 |
| Effects of CH3COOH and K2CO3 on the liquid composition were investigated experimentally | Glucose yield at 200 °C with 0.75 wt% CH3COOH and 10% biomass = 0.855 | ||
| Glucose yield at 220 °C with 0.5 wt% K2CO3 = 0.195 | |||
| Addition of K2CO3 did not suppress hydrolysis in hydrothermal treatment | |||
| Satyanaga-lakshmi et al.18 | Eichhornia crassipes (water hyacinth) | Preliminary pretreatment with different acids (HCl/H2SO4, 2% v/v) and organic acids (acetic/formic acid, 30% v/v) and autoclaving (121 °C, 15 lb) for 60 min and 10 g biomass | Most optimum conditions for pretreatment: 4% w/v H2SO4 pretreatment at 10% w/w biomass loading at temperature of 121 °C for 75 min to produce 0.356 g g−1 reducing sugars |
| Further optimization with H2SO4 concentration: 1–7% w/v; biomass loading 5–30% w/w, temperature 80, 100 and 121 °C; incubation time 15–90 min | Optimum condition for hydrolysis of pretreated biomass: 12.5% w/w biomass loading, incubation period 24 h, surfactant concentration 0.1%, commercial cellulase concentration 70 FPU | ||
| Enzymatic saccharification of biomass with commercial cellulase Zytex (30 FPU per g biomass) and surfactant | Final ethanol yield: 0.292% w/v with actual efficiency of 59.3% |
The approach in the present study is somewhat different from the earlier studies listed in Table 1, which studied the pretreatment of individual biomasses. In a previous paper,2 we presented an extensive study on the assessment and optimization of as many as 17 pretreatment techniques (physical/chemical/physico-chemical) for the invasive species of Parthenium hysterophorus for the maximum production of reducible sugars that could be fermented to produce alcoholic fuels. In the present study, we carried out pretreatment of the five invasive weeds mentioned above at optimized conditions determined for Parthenium hysterophorus,2 and assessed the yield of reducible sugars. It could be expected that the optimum pretreatment conditions for the five invasive weeds could be different than those for Parthenium hysterophorus. The major contemplation underlying the approach of pretreating the invasive weeds listed above at conditions optimized for Parthenium hysterophorus was to assess the output of a bioprocess with feedstock flexibility. This can be explained in greater details as follows: depending on the availability of biomasses in different parts of the year, the biofuel industry for the large-scale production of alcoholic biofuels may require to change the feedstock or to use a mixed feedstock comprising several biomasses as sufficiently large quantities of a single biomass may not be available throughout the year. In such a situation, it may not be feasible or practical to perform comprehensive optimization of the pretreatment conditions for each biomass used as feedstock. Moreover, the optimum pretreatment conditions (such as acid/alkali concentrations or temperature/pressure of the autoclaving) may show significant variations for different biomasses. Thus, the specifications of processing equipment designed for the pretreatment of one biomass may not be suitable for other biomasses. Obviously, the replacement of process equipment for different biomasses is rather impractical and so under this limitation, it is inevitable to treat different biomasses at conditions optimized for the representative biomass that was considered for the process design. In such a situation, it is necessary to make a preliminary estimate of the alterations in the quality of the hydrolyzates in terms of the concentrations of pentose and hexose sugars with changing feedstock. The present study essentially attempts to paint a picture of such variations by pretreatment of the five selected invasive weeds at conditions optimized for the weed of Parthenium hysterophorus.
2. Materials and methods
2.1 Biomass collection and processing
Biomasses of all five weeds, namely AD, SS, MM, LC and EC, were collected from our institute campus. The substrate for pretreatment was the whole plant body except for the roots. After collection, the biomass was dried in ambient air, followed by chopping it into small pieces of a few mm lengths. The chopped biomass was washed with water and again dried in a hot air oven at 60 °C for 24 h. Prior to pretreatment; the particle size of the dried biomass was further reduced to <1 mm using a domestic mixer grinder. The powdered biomass was then stored in air-tight containers at room temperature for further experiments.2.2 Proximate and biochemical analysis of biomass
Total solids, volatile matter, the calorific value and the ash content of all five biomasses were determined according to the standard protocols, namely CEN 15104 and ASTM E1755-01. Ultimate analysis of all the biomass samples was performed by using the Euro EA Elemental (C, H, N) analyzer (Eurovector EA 3000). The results of the proximate and ultimate analyses of the invasive weeds are given in Table 2A. Characterization of the chemical composition (i.e. determination of cellulose, holocellulose and lignin content) of raw, pretreated and delignified biomass was performed using standard protocols, namely Updegreff DM, 1969 and standard TAPPI protocols. The results of the determination of the lignocellulosic composition of the invasive weeds are given in Table 2B.| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Biomass feedstock | Ultimate analysisb | Proximate analysis | |||||
| C (%) | H (%) | N (%) | S (%) | O (%) | Asha (%) | Calorific valuea (MJ kg−1) | |
| a The values are mean ± SE (n = 3).b An approximate variation of ±5% is expected in these values for various samples drawn from same source. | |||||||
| AD | 46.4 | 5.8 | 3.0 | 0.2 | 44.7 | 6.5 ± 0.2 | 18.7 ± 0.4 |
| SS | 49.1 | 6.2 | 2.7 | 0.0 | 41.9 | 9.0 ± 0.9 | 15.7 ± 0.3 |
| MM | 43.1 | 5.6 | 4.6 | 0.0 | 46.7 | 8.5 ± 0.7 | 19.1 ± 0.7 |
| LC | 50.2 | 6.4 | 5.1 | 0.0 | 38.2 | 7.2 ± 0.4 | 18.2 ± 0.3 |
| EC | 41.0 | 5.3 | 4.2 | 0.0 | 49.4 | 20.6 ± 0.1 | 9.21 ± 0.3 |
| (B) | |||||
|---|---|---|---|---|---|
| Sample | Holocellulosea (wt% raw biomass) | Lignin (wt% raw biomass) | Cellulose content of biomass (wt% of biomass after preceding treatment) | ||
| Raw | Post dil. acid hydrolysis | Post delignification | |||
| AD | 69.0 ± 2.8 | 22.5 ± 0.6 | 55.0 ± 4.2 | 74.2 ± 1.2 | 82.3 ± 3.5 |
| SS | 61.0 ± 3.5 | 23.0 ± 3.2 | 49.3 ± 3.0 | 76.6 ± 3.2 | 96.1 ± 3.2 |
| MM | 61.5 ± 1.4 | 19.1 ± 0.7 | 50.2 ± 2.5 | 58.2 ± 3.1 | 93.6 ± 4.3 |
| LC | 61.8 ± 1.9 | 27.2 ± 4.5 | 38.3 ± 3.7 | 69.0 ± 3.4 | 80.7 ± 2.6 |
| EC | 82.2 ± 2.1 | 04.1 ± 0.4 | 47.3 ± 1.7 | 72.7 ± 2.1 | 96.7 ± 3.9 |
2.3 Acid hydrolysis of the biomass
The optimum conditions for the acid hydrolysis of Parthenium hysterophorus were determined by Singh et al.2 as follows: 1% (v/v) H2SO4 (equivalent to 0.36 N) mixed with 10% w/v biomass, then autoclaved at 121 °C and 15 psi for 30 min, followed by rapid steam release. Dried biomass of all five weed species was pretreated under these conditions. After completion of the pretreatment, the biomass from the reaction mixture was separated by filtration through a double-layered muslin cloth. The residual chemicals left on the biomass surface after acid pretreatment were removed by successive water washes. This procedure was continued until the pH of the wash water became 7 (indicating neutral conditions). This was followed by drying of the biomass residue in a hot air oven for 24 h at 60 °C. The dried biomass containing cellulose and traces of lignin was used for further processing. Acid pretreatment causes hydrolysis of the hemicellulose in biomass, resulting in a release of pentose sugars. The filtrate of the acid pretreatment or the acid hydrolyzate was thus analyzed for the sugar content. The hydrolysate was detoxified to remove the inhibitory compounds. The composition of the hydrolyzate was determined using HPLC. The pentose sugars in the hydrolysate predominantly comprise xylose, but other sugars such as arabinose, mannose and galactose are also present; however, their content is negligible as compared to xylose.2.4 Detoxification of acid hydrolyzate
Detoxification of the hydrolyzate from the acid hydrolysis was carried out in two steps. Initially, the pH of the hydrolysate was increased to 10 with the addition of Ca(OH)2, followed by stirring for 30 min. Next, the hydrolyzate was neutralized with the addition of concentrated H2SO4, with subsequent centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min for the removal of suspended solids. 1.5% w/v activated charcoal was added to the hydrolyzate with continuous stirring for 30 min at room temperature. Inhibitory compounds formed during the acid hydrolysis adsorb on the activated charcoal. The particles of activated charcoal were then removed by vacuum filtration of the hydrolyzate.
000 g for 15 min for the removal of suspended solids. 1.5% w/v activated charcoal was added to the hydrolyzate with continuous stirring for 30 min at room temperature. Inhibitory compounds formed during the acid hydrolysis adsorb on the activated charcoal. The particles of activated charcoal were then removed by vacuum filtration of the hydrolyzate.
2.5 Delignification
The delignification of the biomass obtained after acid hydrolysis was carried out using the procedure outlined by Bharadwaja et al.19 This procedure makes use of sonication (or ultrasound irradiation) during delignification. The delignification process is significantly intensified by the physical and chemical effects induced by ultrasound and cavitation. The physical effects of ultrasound and cavitation includes the generation of intense micro-mixing through the phenomena of microstreaming, microturbulence and acoustic waves, while the chemical effects of transient cavitation include the generation of highly reactive radicals through dissociation of gas and vapour molecules entrapped in the bubble.20–23 A probe-type programmable and micro-processor controlled ultrasonic processor (Sonics & Materials, Model VCX 500) with a maximum power of 500 W and frequency of 20 kHz was used for sonication of the reaction mixture. The reaction was carried out in a 100 mL beaker. The total volume of the reaction mixture was 80 mL, with an alkali concentration of 1.5% w/v NaOH and a biomass loading of 2% w/v. The ultrasound probe was set at 30% amplitude, with a theoretical power consumption of 150 W at a duty cycle of 83% (50 s on and 10 s off in 1 min of sonication). The actual power consumption of the ultrasound probe was determined using a calorimetric technique.22,24 The total sonication time was 10 min, during which the temperature of the reaction medium was maintained at 30 °C. After the completion of sonication, the reaction mixture was filtered through a double-layered muslin cloth for removal of the solid biomass residue. This cellulose-rich biomass residue (after removal of the lignin and hemicellulose) was washed with hot water several times until the pH of the wash water was neutral, which ensured no chemicals were left on the biomass surface. The biomass residue was dried for ∼12 h in a hot air oven at 60 ± 3 °C, and was then used for the enzymatic hydrolysis.2.6 Biomass saccharification using commercial enzymes
The enzymatic hydrolysis (or saccharification) of delignified biomasses of all five species was carried out using commercial cellulase and cellobiase enzymes (Sigma Aldrich) at the optimum conditions reported by Bharadwaja et al.19 The hydrolysis was performed in an incubator shaker (Orbitek, Scigenics Biotech) in 50 mM citrate phosphate buffer solution (pH 4.8) at 50 °C and at 150 rpm. The reaction mixture was taken in a 150 mL Erlenmeyer flask with a total reaction volume of 20 mL. The concentration of pretreated biomass in the reaction mixture was 4.2% w/v, with cellulase and cellobiase concentrations of 135 and 75 FPU per g biomass, respectively. The hydrolysis was carried out for 120 h. 0.005% w/v sodium azide solution was added to the mixture to avoid external microbial contamination. 0.1 mL samples of the reaction mixture were withdrawn periodically during hydrolysis and were analyzed to assess the release of sugar.2.7 Determination of reducing sugar in the hydrolyzate
Both pentose-rich acid hydrolyzate and hexose-rich enzyme hydrolyzate were subjected to centrifugation for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (26
000 rpm (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 832 g) at 4 °C. The total reducing sugar in the hydrolyzate was estimated using NS (Nelson and Somogyi) method of. The presence of individual sugars in the hydrolyzate was confirmed through HPLC analysis (Perkin Elmer series 200). The HPLC instrument comprised a pump, a refractive index detector, a vacuum degasser and a Hi-plex-H column (Varian, 300 mm × 5 μm × 4.6 mm). Deionized Milli Q water at a flow rate of 0.4 mL min−1 was used as the mobile phase. Prior to injection in to the HPLC column, samples withdrawn from the reaction mixture were diluted and filtered through a 0.2 μm membrane filter to remove any suspended particulate matter.
832 g) at 4 °C. The total reducing sugar in the hydrolyzate was estimated using NS (Nelson and Somogyi) method of. The presence of individual sugars in the hydrolyzate was confirmed through HPLC analysis (Perkin Elmer series 200). The HPLC instrument comprised a pump, a refractive index detector, a vacuum degasser and a Hi-plex-H column (Varian, 300 mm × 5 μm × 4.6 mm). Deionized Milli Q water at a flow rate of 0.4 mL min−1 was used as the mobile phase. Prior to injection in to the HPLC column, samples withdrawn from the reaction mixture were diluted and filtered through a 0.2 μm membrane filter to remove any suspended particulate matter.
2.8 Characterization of the biomasses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The mixtures were ground well and the spectra were recorded in the range of 400–4000 cm−1 using 200 mg of biomass + a KBr mixture in the form of pellets.
100. The mixtures were ground well and the spectra were recorded in the range of 400–4000 cm−1 using 200 mg of biomass + a KBr mixture in the form of pellets.where Icrystalline = intensity of the crystalline peak at 2θ = 22° and Iamorphous = intensity of the amorphous peak at 2θ = 18°.
3. Results and discussion
3.1 Results of the biomass characterization
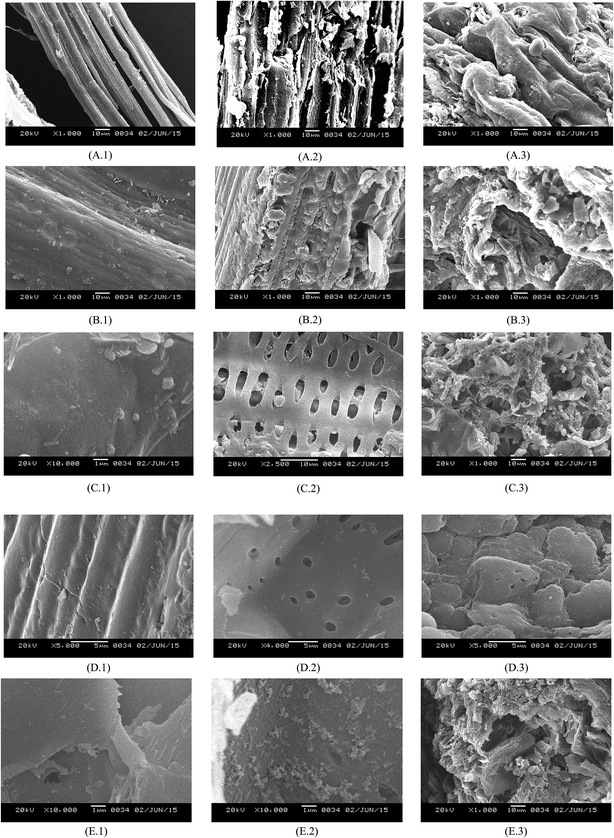
The characterization of the raw biomass and the biomass after two stages of pretreatment, namely acid pretreatment and alkaline delignification, was carried out using three techniques, namely scanning electron microscopy (SEM), FTIR and X-ray diffraction. The results of these analyses are presented below.Irrespective of the type of biomass, the observed effects of pretreatment on the fibre structure of all the biomasses are almost similar. The total amount of residue on the sample surface reduces after pretreatment. Dilute acid pretreatment mainly removes the hemicellulosic fraction. Removal of this fraction creates microspores or holes on the surface of the biomass, as evident from the micrographs shown in Fig. 1C.2 and D.2 corresponding to the biomasses of Mikania micrantha and Lantana camara. Another common feature of the micrographs of all the biomasses is the presence of globular structures on the surface of biomass after dilute acid pretreatment. These structures are associated with lignin condensation and agglomeration. As noted by Lima et al.,26 formation of the globular structures is related to the severity of the pretreatment conditions for that particular biomass. During acid pretreatment at elevated pressure and temperature, the lignin molecules become fluid and coalesce, giving rise to the formation of droplets within the cell matrix. Due to hydrostatic pressure within the cell wall layers, some lignin droplets get redeposited on the biomass surface during cooling of the bulk liquid medium.
The SEM micrographs after alkali treatment or delignification reveal the disappearance of the lignin globules from the biomass surface, with a concurrent rise in the surface roughness of the biomass. This result indicates that alkaline treatment causes degradation of the fibrillar structure or tissue of cellulose and lignin. Destruction of the tissues with removal of the lignin helps in gaining a better access of the cellulose to enzyme action, which results in a faster and higher yield of fermentable sugar from the hydrolysis.
| Band position and assignmentc (cm−1) | Relative change in intensities (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AD AH | AD DLG | SS AH | SS DLG | MM AH | MM DLG | LC AH | LC DLG | EC AH | EC DLG | |
| a Relative change (%) = (intensity of untreated biomass − intensity of pretreated biomass)/intensity of untreated biomass × 100.b Abbreviations: AH-post acid hydrolysis; DLG-post delignification.c Data taken from Kumar et al.,28 Singh et al.,2 Kuhad et al.,12 Sun et al.27 | ||||||||||
| 900 (band of cellulose) | 3.6 | 34.7 | −0.4 | 19.7 | −6.8 | 16.7 | 0.5 | 29.9 | 33.5 | 76.6 |
| 1098 (amorphous to crystalline cellulose ratio) | 2.4 | 27.1 | 11.5 | 16.5 | −10.8 | 2.7 | −6.9 | 15.8 | 32.7 | 74.5 |
1059 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching due to carbohydrate–lignin linkage) O stretching due to carbohydrate–lignin linkage) |
1.8 | 26.4 | 11.9 | 15.7 | −11.7 | 2.3 | −5.3 | 14.1 | 33.4 | 73.7 |
| 1238 (hemicellulose–lignin linkage) | 1.1 | 34.8 | −4.2 | 20.3 | −5.6 | 14.9 | −4.3 | 31.3 | 31.6 | 76.5 |
1245 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption resulting from acetyl group cleavage) O absorption resulting from acetyl group cleavage) |
0.2 | 35.0 | −4.3 | 21.2 | −5.3 | 16.1 | −4.6 | 32.0 | 31.3 | 76.5 |
| 1260 (ester absorbance related to removal of uronic acid) | −0.3 | 36.9 | −0.5 | 26.6 | −5.2 | 18.5 | −5.5 | 33.8 | 31.2 | 77.2 |
| 1378 (band of hemicellulose) | −2.5 | 37.7 | −7.7 | 25.4 | −8.6 | 19.2 | −13.4 | 32.0 | 29.3 | 77.4 |
| 1428 (band of cellulose) | −1.4 | 40.9 | −11.1 | 28.1 | −11.2 | 21.4 | −12.5 | 38.4 | 27.6 | 78.3 |
| 1458 (aromatic ring vibration related to lignin removal) | −4.0 | 19.6 | 5.4 | 9.0 | −8.0 | −1.3 | −11.0 | 13.0 | 25.7 | 68.9 |
| 1508 (aromatic ring vibration related to lignin removal) | −6.1 | 15.2 | −2.5 | 1.2 | −8.3 | −7.8 | −14.7 | 8.1 | 25.1 | 65.7 |
| 1595 (aromatic ring stretch related to lignin removal) | −4.3 | 30.3 | −3.4 | 19.8 | −11.5 | 7.6 | −20.1 | 26.2 | 25.5 | 75.3 |
| 1720 (carboxylic acids/ester groups) | −5.9 | 27.7 | −23.6 | 11.7 | −0.8 | 14.3 | −6.2 | 30.1 | 26.7 | 72.8 |
1738 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching due to carbohydrate linked with lignin) O stretching due to carbohydrate linked with lignin) |
−9.0 | 28.6 | −31.5 | 12.5 | −4.1 | 16.1 | −9.6 | 32.1 | 24.0 | 72.7 |
| 1745 (carbonyl bonds related to lignin side chain removal) | −11.9 | 29.2 | −40.5 | 11.1 | −6.0 | 15.0 | −12.7 | 33.3 | 22.9 | 72.8 |
| 2900 (C–H stretching related to rupture of methyl/methylene group of cellulose) | 11.5 | 37.2 | −21.5 | 25.5 | −5.2 | 20.9 | −15.5 | 40.7 | 18.8 | 76.5 |
| 3348 (O–H stretching related to rupture of cellulose–hydrogen bonds) | 14.3 | 17.6 | −14.8 | 9.6 | −11.1 | −9.4 | −21.7 | 20.8 | 18.2 | 74.9 |
As per the above definition, a positive relative percentage change of intensity at all specific bands indicates a reduction in the particular component assigned to that band. The IR spectra of biomasses at three stages, namely raw biomass, post dilute acid pretreatment and post delignification, are given in the ESI.† For most absorption bands, the values of the relative change in the intensity were revealed to be positive after completion of both pretreatments. The changes indicate the efficient removal of hemicellulose and lignin after the biomass treatments. Positive changes for the band positioned at 1378 cm−1 indicates the removal of hemicellulose during acid hydrolysis. Positive changes for the band positioned at 900 and 1098 cm−1 indicate a reduction in cellulose crystallinity, which assists in enzymatic hydrolysis. The overall conclusion of the FTIR analysis is that the treatment of acid hydrolysis at 1% v/v H2SO4, 121 °C and 15 psi pressure, and alkaline delignification at 1.5% w/v NaOH assisted by ultrasound are able to induce desired changes in the biomass structure; although these conditions have not been individually optimized for each of the five biomasses considered in the present work.
| Biomass | Crystallinity index, CrI (%) | ||
|---|---|---|---|
| Raw | Post dil. acid hydrolysis | Post delignification | |
| AD | 37.5 | 60 | 64.70 |
| SS | 52.00 | 56.52 | 62.50 |
| MM | 20.00 | 38.70 | 48.57 |
| LC | 50.00 | 47.50 | 57.14 |
| EC | 16.67 | 33.33 | 42.85 |
The crystallinity index shows a further rise with alkaline delignification. Alkali pretreatment in the presence of ultrasound leads to delignification, with saponification of the intermolecular ester bonds cross-linking the xylan hemicelluloses and lignin, in addition to breakage of the linkages between cellulose and lignin. Singh et al.2 observed that the alkaline treatment of lignocellulosic biomass results in a swelling of the biomass, also with an increase in surface area, a decrease in the degree of polymerization, separation of the structural linkages between lignin and carbohydrates and disruption of the lignin structure. All of these effects contribute to a reduction in the amorphous fraction of biomass and exposure of the crystalline cellulose fraction, which is reflected in an increase in crystallinity of all the five biomasses after delignification. These results are in concurrence with the results of Singh et al.,2 who observed an increase in the crystallinity of Parthenium hysterophorus after 1% v/v H2SO4 treatment at 121 °C and 15 psi pressure and delignification with 1.5% w/v NaOH treatment in the presence of ultrasound. The results of the X-ray diffraction analysis are additional corroborations that, although conditions for the dilute acid hydrolysis and delignification are not optimized for the five invasive weeds used in the present study, the pretreatment of the weed biomasses at these conditions was still able to induce desired changes in their composition.
3.2 Results of dilute acid and enzymatic hydrolysis
The experimental results on the pretreatment and enzymatic hydrolysis of the five biomass species considered in this work are given in Tables 2B and 5A. It should be noted that in Table 2B, the cellulose content of biomass after dilute acid hydrolysis is expressed as a percentage of raw biomass, while that after delignification is expressed as a percentage of biomass after acid pretreatment.| (A) | ||||||
|---|---|---|---|---|---|---|
| Biomass | TRS released in AH (g L−1) | TRS yield in AH (mg g−1 of raw biomass) | TRS released in EH till 36 h (g L−1) | TRS yield in EH till 36 h (mg g−1 of delignified biomass)b | TFS (mg g−1 of raw biomass) | TFS yield (g per 100 g of raw biomass) |
| a TRS – total reducing sugar; TFS – total fermentable sugar, AH – acid hydrolysis; EH – enzymatic hydrolysis.b For greater details on enzymatic hydrolysis of invasive weeds, refer to Borah et al.29c Results in Table 2B indicate that for biomasses of AD and LC, complete delignification is not achieved at treatment conditions of 1.5% w/v NaOH and sonication.d Rate of TRS release is calculated ignoring the traces of lignin left in the biomass after alkaline treatment with sonication.e Maximum theoretical yield for ethanol from hexose as well as pentose sugars is 0.51 g g−1 sugar.2f Maximum theoretical yield for butanol from hexose as well as pentose sugars is 0.41 g g−1 sugar.2 | ||||||
| AD | 31.60 | 315.9 | 30.41 | 724.0 | 492.9 | 49.29 |
| SS | 39.45 | 394.5 | 35.81 | 851.7 | 585.9 | 58.59 |
| MM | 27.21 | 272.1 | 24.90 | 592.0 | 449.7 | 44.37 |
| LC | 11.80 | 118.0 | 27.81 | 662.2 | 306.7 | 30.66 |
| EC | 23.86 | 238.6 | 31.86 | 758.6 | 363.6 | 36.36 |
For the biomass of Saccharum spontaneum and Eichhornia crassipes, the percentage cellulose content of biomass after pretreatment is above 95%, indicating almost the complete removal of lignin and hemicellulose. This was also confirmed by the large positive values for the percentage relative changes to various bands in the IR spectrum of Saccharum spontaneum and Eichhornia crassipes (as depicted in Table 3) corresponding to different functional groups in the biomasses. However, for the biomasses of Arundo donax and Lantana camara, the percentage cellulose content after delignification and acid hydrolysis was less then 85%, which indicates the presence of residual lignin in biomass after pretreatment. The results given in Table 2B and demonstrate the potentials of the weed species for biofuel production. The holocellulose content of Eichhornia crassipes is the highest, while Lantana camara has the highest lignin content. The lignin hinders the hydrolysis of hemicellulose during dilute acid pretreatment. The subsequent alkali pretreatment at 1.5% w/v NaOH assisted with sonication has also not been able to remove the lignin completely. As a consequence, the pretreated biomass undergoing enzymatic hydrolysis comprises only 80% cellulose. An interesting result was seen for the biomasses Arundo donax and Saccharum spontaneum, where the initial lignin content of these biomasses is almost similar, yet the biomass of Saccharum spontaneum after pretreatment had a far higher content of cellulose than Arundo donax. An explanation for this discrepancy can be given in terms of the structural composition of lignin in Arundo donax. You et al.7 recently published an analysis of the lignin structure from the stems and foliage of Arundo donax, and described how lignin is an amorphous polymer, and is made up of three aromatic alcohols or monolignols, namely p-coumaryl, coniferyl and syringyl. These monolignols form distinct lignin units during the lignification process, known as p-hydroxy phenyl (H), guaiacyl (G) and syringyl (S) units. The analysis of You et al.7 clearly indicated that the milled wood lignin (both stem and foliage) of Arundo donax were HGS-type lignin with an S/G ratio in the range of 0.15–0.62, with a strong predominance of G units. The main lignin interunit linkages were β-O-4 alkyl–aryl ethers followed by β–β′, β-1′ and α,β-diaryl ethers together with cinnamyl alcohol and cinnamaldehyde end groups. The foliage lignin with higher condensed G units contained a greater amount of tricin. The linkages between lignin and tricin could be alkaline stable. As a result of its structural characteristics, the lignin in Arundo donax is not removed completely during alkaline pretreatment at 1.5% w/v NaOH.
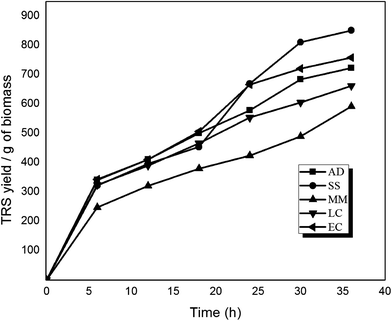 | ||
| Fig. 2 Time profile of TRS release during enzymatic hydrolysis of delignified biomasses of different invasive weeds for 36 h of treatment. | ||
Some other observations on the results of the enzymatic hydrolysis and their plausible explanations on the basis of characterization of the biomasses are as follows:
(1) Delignification causes depolymerization of lignin through homolytic cleavages of the phenyl ether β-O-4 and α-O-4 bonds, resulting in exposure of the cellulose moieties in the biomass. The residual biomass after delignification is rich in cellulose and has an increased crystallinity due to removal of the amorphous lignin and hemicellulose. However, we find that the order of crystallinity of delignified biomass (i.e. AD > SS > LC > MM > EC) does not follow the order of the cellulose content of biomass (i.e. EC > SS > MM > AD > LC). This disparity is attributed to two factors: (1) loss of crystallinity during acid hydrolysis due to partial hydrolysis of the cellulose (in addition to hemicellulose), as noted earlier, and (2) the presence of residual lignin in biomass after pretreatment.
(2) The lesser the crystallinity of the cellulose, the faster its reactivity during enzymatic hydrolysis, thus resulting in high hexose sugar yields. However, the order of sugar yields in enzymatic hydrolysis (i.e. SS > EC > AD > LC > MM) does not follow the order of the post-delignification crystallinity of the biomass (i.e. AD > SS > LC > MM > EC). This disaccord is also ascribed to other factors affecting the reaction between the enzymes and the cellulose substrate, such as the presence of residual lignin in the biomass, which may compete with cellulose for adsorption of the enzyme, and hindrance offered by the ash content of the cellulose.
3.3 Assessment of biofuels production potential
The potential of the five invasive weeds as feedstock for biofuels can be assessed on the basis of the maximum theoretical production of alcoholic biofuels, such as ethanol and butanol from fermentation of the hydrolyzates resulting from acid pretreatment and enzymatic hydrolysis. The theoretical yields of ethanol and butanol resulting from the total fermentable sugars (inclusive of both the pentose and hexose hydrolyzates obtained from the acid and enzymatic treatments) from each of the five invasive weeds are listed in Table 5C. It can be seen that the biomass of Saccharum spontaneum had a maximum yield of 29.88 g ethanol and 24.02 g butanol per 100 g of raw biomass. The least yields of 15.63 g ethanol and 12.51 g butanol per 100 g of raw biomass were observed for the biomass of Lantana camara. For the biomass of Mikania micrantha, which is a relatively novel feedstock for bioalcohol production, the theoretical yields were 22.62 g ethanol and 18.19 g butanol per 100 g of raw biomass. The average yield of ethanol from all five invasive weeds was 22.36 g per 100 g of raw biomass, while the average yield of butanol was 17.96 g per 100 g of raw biomass. These yields are at par with the yields reported by Singh et al.3 for Parthenium hysterophorus, i.e. 20 g ethanol per 100 g raw biomass and 16 g butanol per 100 g raw biomass. The similar yields of ethanol and butanol for the five invasive weeds, as compared to Parthenium hysterophorus, are indicative of the promising potential of these weeds for biofuels production – even under un-optimized pretreatment conditions for each of the biomass. This result also highlights that these invasive weeds could be used as multiple feedstocks in a biorefinery, and could be expected to support an overall consistent and sustainable production of alcoholic biofuels. For the efficient production of alcoholic biofuels with a maximum yield per unit raw biomass, it is essential that the hydrolyzates from both the acid and enzymatic treatments can be utilized for fermentation. These hydrolyzates could be fermented separately (as demonstrated by Bharadwaja et al.19) or simultaneously using mixed cultures. The theoretical bioalcohol yields from the five tested invasive weeds (with a proper utilization of pentose and hexose sugars) are on par with the yields from conventional fermentation substrates, such as corn and molasses, and also second-generation lignocellulosic substrates, such as agro-residues of rice/wheat crops. However, the invasive weeds being waste biomasses (with no other outlet such as cattle feed or domestic fuel) are likely to be available at far cheaper prices than agro-residues. Thus, the processes for biofuels production with invasive weeds as a feedstock are expected to offer significantly lower production costs and contribute to an attractive economy that should interest the stakeholders in the biofuels industry.4. Conclusion
The present study investigated the bioalcohol production potential of five invasive weeds, namely Arundo donax, Saccharum spontaneum, Mikania mikrantha, Lantana camara and Eichhornia crasspies in terms of the yield of total fermentable sugars from pretreatment and enzymatic hydrolysis. The pretreatment comprised two steps: dilute acid hydrolysis and alkaline delignification with sonication, followed by enzymatic hydrolysis of the pretreated biomass. These pretreatments were carried out at conditions optimized for the waste biomass of Parthenium hysterophorus. Thus, the pretreatment conditions were not optimized specifically for any of the five biomasses considered in this study. Despite this limitation, the average TFS yield from the five invasive weeds was 43.85 g per 100 g of raw biomass, which is on par with the yield from Parthenium hysterophorus for optimized pretreatment conditions. This TFS yield corresponded to a (theoretical) bioethanol yield of 22.36 g and a biobutanol yield of 17.96 g per 100 g of raw biomass. These results vividly demonstrate that a consistent production of alcoholic biofuels could be achieved from the use of invasive weeds as multiple feedstocks, even if the pretreatment conditions are not optimized for each of the weeds. The results of the present study have also demonstrated the feasibility of the large-scale production of alcoholic biofuels employing multiple biomass feedstocks of different invasive weeds.Acknowledgements
The authors gratefully acknowledge use of SEM facility at Sophisticated Analytical Instruments Facility (SAIF), Northeastern Hill University (NEHU), Shillong. Use of XRD facility (procured through FIST grant No. SR/FST/ETII-028/2010 from Department of Science and Technology, Government of India) and the HPLC and FTIR facility at Department of Chemical Engineering, IIT Guwahati is also acknowledged. One of the authors (AJB) acknowledges help in the experimental work from Mr S. T. P. Bharadwaja. Authors would like to thank the anonymous referees of the manuscript for their meticulous evaluation and constructive criticism.References
- A. Ranjan, S. Khanna and V. S. Moholkar, Appl. Energy, 2013, 103, 32–38 CrossRef CAS.
- S. Singh, S. Khanna, V. S. Moholkar and A. Goyal, Appl. Energy, 2014, 129, 195–206 CrossRef CAS.
- A. Ekman, O. Wallberg, E. Joelsson and P. Borjesson, Appl. Energy, 2013, 102, 299–308 CrossRef CAS.
- J. van Eijck, B. Batidzirai and A. Faaij, Appl. Energy, 2014, 135, 115–141 CrossRef.
- T. Komolwanich, P. Tatijarern, S. Prasertwasu, D. Khumsupan, C. Thanyalak, A. Luengnaruemitchai and S. Wongkasemjit, Cellulose, 2014, 21, 1327–1340 CrossRef CAS.
- D. Scordia, S. L. Cosentino and T. W. Jeffries, Ind. Crops Prod., 2013, 49, 392–399 CrossRef CAS.
- T. You, L. Zhang, S. Zhou and F. Xu, Bioresour. Technol., 2014, 167, 574–577 CrossRef CAS PubMed.
- W. F. Anderson, B. S. Dein, S. K. Brandon and J. D. Peterson, Appl. Biochem. Biotechnol., 2008, 145, 13–21 CrossRef CAS PubMed.
- D. Scordia, S. L. Cosentino, J. W. Lee and T. W. Jeffries, Biomass Bioenergy, 2011, 35, 3018–3024 CrossRef CAS.
- A. Kuila and R. Banerjee, Bioprocess Biosyst. Eng., 2014, 37, 1963–1969 CrossRef CAS PubMed.
- R. Gupta, Y. P. Khasa and R. C. Kuhad, Carbohydr. Polym., 2011, 84, 1103–1109 CrossRef CAS.
- R. C. Kuhad, R. Gupta, Y. P. Khasa and A. Singh, Bioresour. Technol., 2010, 101, 8348–8354 CrossRef CAS PubMed.
- R. Kataria and S. Ghosh, Energy Sources, Part A, 2014, 36, 1028–1035 CrossRef CAS.
- G. Chaudhury, L. K. Singh and S. Ghosh, Bioresour. Technol., 2012, 124, 111–118 CrossRef PubMed.
- A. K. Chandel, O. V. Singh, L. V. Rao, G. Chandrasekhar and M. L. Narasu, Bioresour. Technol., 2011, 102, 1709–1714 CrossRef CAS PubMed.
- J. Yan, W. Zhilei, Q. Wang, M. H. He, S. Li and C. Irbis, Bioresour. Technol., 2015, 193, 103–109 CrossRef CAS PubMed.
- P. P. Phothisantikul, R. Tuanpusa, M. Nakashima, T. Charinpanitkul and Y. Matsumura, Ind. Eng. Chem. Res., 2013, 52, 5009–5015 CrossRef CAS.
- K. Satyanagalakshmi, R. Sindhu, P. Binod, K. U. Janu, R. K. Sukumaran and A. Pandey, J. Sci. Ind. Res. India, 2011, 70, 156–161 CAS.
- D. M. Updegraff, Anal. Biochem., 1969, 32, 420–424 CrossRef CAS PubMed.
- S. T. P. Bharadwaja, S. Singh and V. S. Moholkar, J. Taiwan Inst. Chem. Eng., 2015, 51, 71–78 CrossRef.
- H. A. Choudhury, R. S. Malani and V. S. Moholkar, Chem. Eng. J., 2013, 231, 262–272 CrossRef CAS.
- R. Patidar, S. Khanna and V. S. Moholkar, Ultrason. Sonochem., 2012, 19, 104–118 CrossRef CAS PubMed.
- S. Chakma and V. S. Moholkar, AIChE J., 2013, 59, 4303–4313 CrossRef CAS.
- V. S. Moholkar and M. M. C. G. Warmoeskerken, AIChE J., 2003, 49(11), 2918–2932 CrossRef CAS.
- T. Sivasankar, A. W. Paunikar and V. S. Moholkar, AIChE J., 2007, 53, 1132–1143 CrossRef CAS.
- L. Segal, J. J. Creely, A. E. Martin Jr and C. M. Conrad, Text. Res. J., 1962, 29, 786–794 CrossRef.
- M. A. Lima, G. B. Lavorente, H. K. P. Da Silva, J. Bragatto, C. A. Rezende, O. D. Bernardinelli, E. R. De Azevedo, L. D. Gomez, S. J. McQueen-Mason, C. A. Labate and I. Polikarpov, Biotechnol. Biofuels, 2013, 6, 75 CrossRef CAS PubMed.
- X. F. Sun, F. Xu, R. C. Sun, P. Fowler and M. S. Baird, Carbohydr. Res., 2005, 340, 97–106 CrossRef CAS PubMed.
- R. Kumar, G. Mago, V. Balan and C. E. Wyman, Bioresour. Technol., 2009, 100, 3948–3962 CrossRef CAS PubMed.
- A. J. Borah, M. Agarwal, M. Poudyal, A. Goyal and V. S. Moholkar, Bioresour. Technol., 2016 DOI:10.1016/j.biortech.2016.02.024.
Footnote |
| † Electronic supplementary information (ESI) available: (1) FTIR spectra of native or raw biomass, biomass post dilute acid hydrolysis and biomass post alkaline delignification. (2) X-ray diffractograms of five biomass species (native biomass and the biomass after different pretreatments). See DOI: 10.1039/c5ra27787f |
| This journal is © The Royal Society of Chemistry 2016 |