Multivalent photo-crosslinkable coumarin-containing polybenzoxazines exhibiting enhanced thermal and hydrophobic surface properties†
Abstract
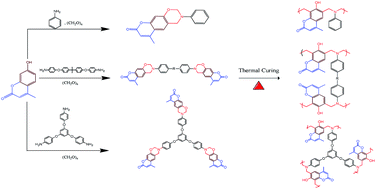
In this study, mono-, bi-, and trivalent coumarin-containing benzoxazine monomers (mono-, di-, and tri-coumarin BZ) were synthesized in high yield and purity by facile Mannich reactions of 4-methyl-7-hydroxycoumarin and paraformaldehyde with aniline, bisphenol A–NH2, and 1,3,5-tri(4-aminobenzene), respectively, in 1,4-dioxane. 1H and 13C nuclear magnetic resonance (NMR), Fourier transform infrared (FTIR) and high resolution mass spectroscopy support the chemical structures of these three benzoxazine monomers. Differential scanning calorimetry (DSC) and FTIR spectroscopy were used to investigate the curing polymerization behavior and photodimerization ([2π + 2π] cycloaddition) of the coumarin units of mono-, di-, and tri-coumarin BZ to form poly(mono-coumarin BZ), poly(di-coumarin BZ), and poly(tri-coumarin BZ), respectively. DSC measurement revealed that the thermal polymerization temperature of coumarin-containing benzoxazine monomers was lower than that of the model compound 3-phenyl-3,4-dihydro-2H-benzooxazine (263 °C) which was attributed to the catalytic effect of the coumarin moiety and a strong electron withdrawing electron conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the coumarin unit. In addition, the glass transition and thermal decomposition temperatures of poly(tri-coumarin BZ) (Tg = 240 °C; Td5 = 370 °C) were higher than poly(di-coumarin BZ) and poly(mono-coumarin BZ), consistent with the former's higher crosslinking density. In addition, the water contact angles of poly(tri-coumarin BZ) polymers prepared with and without photo-dimerization prior to thermal curing (112 and 110°, respectively) were higher than the corresponding poly(mono-coumarin BZ) and poly(di-coumarin BZ), presumably because of greater degrees of intramolecular hydrogen bonding between the C
C bond in the coumarin unit. In addition, the glass transition and thermal decomposition temperatures of poly(tri-coumarin BZ) (Tg = 240 °C; Td5 = 370 °C) were higher than poly(di-coumarin BZ) and poly(mono-coumarin BZ), consistent with the former's higher crosslinking density. In addition, the water contact angles of poly(tri-coumarin BZ) polymers prepared with and without photo-dimerization prior to thermal curing (112 and 110°, respectively) were higher than the corresponding poly(mono-coumarin BZ) and poly(di-coumarin BZ), presumably because of greater degrees of intramolecular hydrogen bonding between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units of the coumarin moieties and the phenolic OH units of the benzoxazine rings, resulting in lower surface free energies. Thus, the presence of multivalent photo-crosslinkable coumarin units enhanced the thermal and hydrophobic surface properties of these polybenzoxazines.
O units of the coumarin moieties and the phenolic OH units of the benzoxazine rings, resulting in lower surface free energies. Thus, the presence of multivalent photo-crosslinkable coumarin units enhanced the thermal and hydrophobic surface properties of these polybenzoxazines.

 Please wait while we load your content...
Please wait while we load your content...