Light-controlled bubble propulsion of amorphous TiO2/Au Janus micromotors†
Yan Li,
Fangzhi Mou*,
Chuanrui Chen,
Ming You,
Yixia Yin,
Leilei Xu and
Jianguo Guan*
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 122 Luoshi road, Wuhan 430070, P. R. China. E-mail: moufz@whut.edu.cn; guanjg@whut.edu.cn
First published on 15th January 2016
Abstract
In this work, a bubble-propelled photoactivated amorphous TiO2/Au (Am-TiO2/Au) Janus micromotor has been demonstrated by utilizing the efficient photocatalytic H2O2 decomposition over the in situ H2O2 sensitized Am-TiO2 under UV irradiation. The power conversion of the micromotor experiences a process from UV light power, to chemical power and finally to mechanical power. The quantum efficiency of O2 bubble evolution from photocatalytic decomposition of H2O2 reaches 28%, and the power conversion efficiency reaches 1.28 × 10−9, which enables the micromotor to generate a strong bubble thrust propelling itself forward with a maximum speed of 135 μm s−1. The motion state and speed of the Am-TiO2/Au micromotors can be reversibly, wirelessly and remotely controlled at will with an ultrafast response rate (less than 0.1 s) by regulating “on/off” switch and intensity of UV irradiation. Consequently, the as-developed Am-TiO2/Au micromotors may promise potential challenging applications in such as “on-the-fly” adsorption and decomposition of pollutants in water.
Introduction
Micro/nanomotors could harvest energy from the environment and transform it into autonomous motions.1–11 Due to their fascinating capabilities to pick up, transport, and release various micro/nanocargoes in liquid media, the micro/nanomotors manifest a great potential in paramount applications in drug delivery, cell separation, microsurgeries, lithography and environmental remediation.12–21 To meet the demands of these envisioned applications, the motions of micro/nanomotors should be regulated over both defined times and locations, including controllable activation/stop, motion direction and speed.22 Up to now, various motion controlling strategies have already been demonstrated to control the motions of the micro/nanomotors, including magnetic field,19,23,24 light,13,25–29 heat pulse,30–32 ultrasound field33,34 and electric field.35 Among them, light is one of the most powerful and versatile physical triggers, and can be remotely applied with extremely high spatial and temporal precision. It exhibits outstanding advantages for controlling the micromotors.Several pioneer works have demonstrated the light triggered “on/off” motions of micro/nanomotors by taking advantages of photothermal effect or photocatalytic reactions. But using photothermal energy to manipulate micro/nanomotors usually has a trouble of long response time, such as in activation or stop etc., because of the limited thermal conductivity of aqueous fuels (about 0.65 W m−1 K−1).30,32 Photocatalytic reactions can be instantly triggered under light irradiation, and is a promising underlying propulsion mechanism for the light-controlled micromotors.26–28 For instance, the light-controlled TiO2 tubular microengines and TiO2/Pt Janus micromotors have been developed by employing the photocatalytic decomposition of H2O2 and water respectively over the well-crystallized anatase TiO2.13,29 Compared with the crystalline TiO2, amorphous TiO2 (Am-TiO2) has a long term disordered but short term ordered structure, and possesses obvious advantages, including the simple preparation at room-temperature and much higher surface area.36 Although pure Am-TiO2 has long been reported to be nearly inactive due to the unfavorable recombination of photogenerated electrons and holes under UV irradiation,37 the H2O2-sensitized Am-TiO2 recently has been reported to exhibit a superior photocatalytic activity to the crystalline TiO2 owing to the enhanced separation of the photogenerated charge pairs by the surface peroxide complexes.38–40 Hence, Am-TiO2 has the potential to photocatalytically decompose H2O2 to generate massive O2 bubbles due to the in situ H2O2 sensitization, which is promising for remote activation and propulsion of micromotors.
In this work, we for the first time demonstrate a bubble-propelled photoactivated Am-TiO2/Au Janus micromotors by utilizing the efficient photocatalytic H2O2 decomposition over the in situ H2O2 sensitized Am-TiO2 under UV irradiation. For the as-developed Am-TiO2/Au micromotors, the motion state and speed can be reversibly, wirelessly and remotely controlled at will with an ultrafast response rate (less than 0.1 s) by regulating “on/off” switch and intensity of UV irradiation. In comparison with the light-controlled micro/nanomotors consisting of crystallized phases,13,29 those with amorphous structures are expected to have a few potential advantages, including simple preparation and a much higher surface area for chemical doping or adsorption. Consequently, the as-developed Am-TiO2/Au micromotors are expected to have potential applications in such as “on-the-fly” adsorption and decomposition of pollutants in water.
Experimental
Preparation of Am-TiO2/Au Janus microspheres
Am-TiO2 microspheres were firstly prepared by an O/W microemulsion method. Briefly, 2 mL caprylic acid (CA) solution with 10 wt% of tetrabutyl titanate (TBT) as an oil phase was added into 40 mL polyvinyl alcohol (2 wt%) aqueous solution. The above mixed solutions were magnetically stirred at a rate of 500 rpm for 2 min to form TBT/CA microdroplets in the aqueous solution. Then, 4.5 mL ammonia (25 wt%) solution was dropped into the O/W emulsion to trigger the hydrolysis of TBT/CA droplets, and the solution was stirred for another 5 min. After standing for 24 h, the Am-TiO2 microspheres were formed. The Am-TiO2 microspheres were dispersed in ethanol after centrifuged and washed with deionized water for 8 times and ethanol 3 times.To prepare Am-TiO2/Au Janus microspheres, 200 μL of the Am-TiO2 microspheres suspension was dropped on a glass slide and dried at 80 °C for 5 min. Then, the exposed surfaces of the Am-TiO2 microspheres were coated with a gold layer via ion sputtering for 240 s (about 35 nm in thickness) under a pressure of 0.6 Pa. The Am-TiO2/Au Janus microspheres were then separated from the glass slide by an ultrasonication process.
Characterization
Scanning electron microscopy (SEM), and energy-dispersive X-ray (EDX) analysis were obtained using a Hitachi S-4800 field-emission SEM (Japan). X-ray diffraction pattern (XRD) was obtained by using a Rigaku D/Max-RB diffractometer at a voltage of 35 kV and a current of 30 mA with Cu-Kα radiation (λ = 0.15406 nm).Recording microscopy videos and analysis
20 μL Am-TiO2/Au Janus microspheres aqueous suspension was added to 1 mL of the fuel solution in a Petri dish with a diameter of 35 mm. UV-LED light source was placed at 25 mm above the surface of the fuel solution, and the UV intensity on the surface of the fuel solution is 1 W cm−2. The motions of the Am-TiO2/Au Janus microspheres upon UV irradiation with different intensities were observed and recorded at room temperature through an optical microscope (Olympus BX60). All videos of the micromotor movement were analyzed using Video Spot Tracker V08.01 software.Results and discussion
The Am-TiO2/Au Janus micromotors are prepared by asymmetrically coating Au layer on the exposed surface of Am-TiO2 microspheres (Fig. S1†) on a flat glass substrate via ion sputtering process. Fig. 1A shows that Am-TiO2/Au Janus micromotors have an average diameters of about 15 μm. The elemental linear (Fig. 1B) confirms the binary heterostructure of the micromotor, in which about 3/4 of the surface of TiO2 microsphere is covered by an asymmetric spherical-cap of Au layer. The broad and weak XRD peaks in Fig. 1C confirm that TiO2 in the micromotors have an amorphous structure, while Au peaks can not be detected due to the low content of Au in the micromotors. | ||
| Fig. 1 The SEM image (A), linear EDX analysis (B) and XRD pattern of the Am-TiO2/Au Janus microspheres. | ||
The efficient photocatalytic H2O2 decomposition over the in situ H2O2 sensitized Am-TiO2 under UV irradiation plays a crucial role in the light-controlled propulsion of the Janus micromotor. Am-TiO2, which has a long term disordered but short term ordered structure, has long been reported to be nearly inactive due to the facilitated recombination of photogenerated electrons and holes under UV irradiation because of their high density of defects. However, the Am-TiO2 sensitized with H2O2 exhibits an efficient photocatalytic activity even higher than the crystalline TiO2 and commercial Degussa P25 owing to the formation of surface peroxide complexes on the amorphous TiO2 surface and its effect for the separation photogenerated electrons and holes.38–40 When Am-TiO2/Au micromotors are added into the H2O2 fuels, the Am-TiO2 component is in situ sensitized by H2O2, generating peroxide complexes on their surfaces. Under UV irradiation, the photoexcited electrons in Am-TiO2 transfer to the surface peroxide complexes and leaves holes behind thanks to its strong affinity for electrons, prolonging the life time of the photogenerated holes and electrons to participate in the photocatalytic H2O2 decomposition according to eqn (1)–(3).
 | (1) |
| H2O2 + 2h+ → O2 + 2H+ | (2) |
| H2O2 + 2e− + 2H+ → 2H2O | (3) |
The O2 bubbles generated from the photocatalytic decomposition of H2O2 could then be selectively ejected from the naked Am-TiO2 surface to propel the micromotor. As shown in ESI-Video 1† and Fig. 2, the Am-TiO2/Au Janus micromotor exhibits an efficient photoactivated motion in 15 wt% H2O2 with 5 wt% surfactant (Triton-X100). Once the UV light (wavelength λ = 368 nm) is on, a long tail of bubbles with diameter (R) of ca. 10 μm generated on one side of the Am-TiO2/Au Janus micromotor, which engender a strong momentum that propels the micromotor forward (Fig. 2A and D) with a remarkable speed of 135 μm s−1 (over 6 body lengths per second). When the UV light is off, the bubble generation and the motion of the micromotor stops immediately (Fig. 2B). The stopped micromotor can be re-activated within 0.1 s if the UV light is turned back on (Fig. 2C), reflecting the fast response of the micromotor on UV irradiation. This “on/off” propulsion of the micromotor is reversible by turning on or off the UV irradiation, as shown in ESI-Video 1.† Most of the Am-TiO2/Au Janus micromotors exhibit anticlockwise (Fig. 2) or clockwise (Fig. S2†) rotational motions due to the rotational torque generated from the deviation of the direction of the propulsive force with the centroid of the micromotors. The Am-TiO2/Au Janus micromotors show a limited lifetime of about 20 min based on our observation because the Am-TiO2 could be gradually dissolved by concentrated H2O2 solution. However, this life time is much longer than that of the micromotors based on etching reaction of active metals.16,41–43
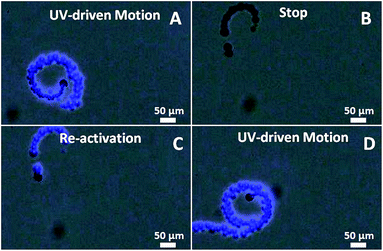 | ||
| Fig. 2 The lighted-controlled propulsion of a typical Am-TiO2/Au Janus micromotor (ESI-Video 1†) in the fuel solution with 15 wt% H2O2 and 5 wt% Triton-X100 at a time interval of (A) 0, (B) 3, (C) 3.1, and (D) 5 s. | ||
The intensity (I) of the UV irradiation on the micromotors is measured to be 1 W cm−2. The photon flux (Φ) of the UV source can be calculated to be 1.85 × 1022 photons per m2 per second according to eqn (4), in which h, c and λ represent Planck's constant (6.626 × 10−34 J s), speed of light (3 × 108 m s−1) and wavelength (368 nm) of the UV light, respectively.
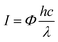 | (4) |
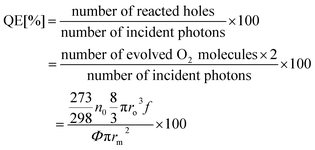
The apparent quantum efficiency (QE) of O2 bubbles from the micromotor driven by photocatalytic decomposition of H2O2 can be calculated according to eqn (5).44
 | (5) |
The power conversion of Am-TiO2/Au Janus micromotor experiences a process from UV light power, to chemical power and finally to mechanical power. The mechanical power of the micromotor is in form of kinetic power, which is harvested from the motion of the micromotor under the recoil force of the growth, ejection and burst of the generated O2 bubbles.45 The power conversion efficiency (ηc) of the UV-driven micromotor, which can be defined as the ratio of the output mechanical power (Pmecha = 6πμrmv2 = 3.15 × 10−15 W) into the input overall chemical power (Pchem = NΔrGθ = 2.08 × 10−7 W) and UV light power (PUV = Iπrm2 = 2.27 × 10−6 W), can be estimated from eqn (6).46
 | (6) |
To confirm the photocatalytic propulsion mechanism of the micromotor, the generation of O2 bubbles on the naked Am-TiO2 microspheres is also examined. When Am-TiO2 microspheres are added into the H2O2 solution, the white microspheres turned into yellow ones, suggesting the formation of peroxide complexes on their surfaces. As shown in ESI-Video 2† and Fig. 3A and B, masses of bubbles are generated from the entire surface of naked Am-TiO2 microspheres in the H2O2 fuel when UV light is turned on, while bubble generation stops when UV light is turned off, suggesting fast photocatalytic decomposition of H2O2 over Am-TiO2 under UV irradiation. No directional movement can be observed for naked Am-TiO2 microspheres in H2O2 fuel under UV irradiation, indicating the symmetrically released bubbles from the microspheres do not contribute to propulsion.
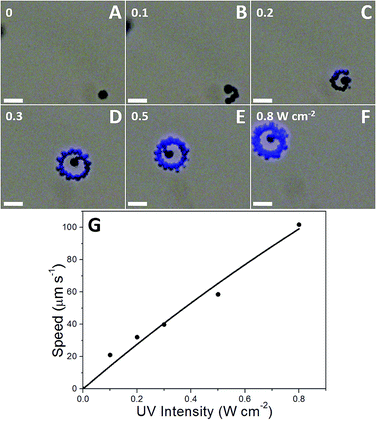
It has been reported that coupling TiO2 with Au could improve the separation of the photogenerated electrons and holes, and enhance the photocatalytic activity of the TiO2 afterwards.47 However, the bubble generation rate on the naked Am-TiO2 microspheres (Fig. 3) is similar to that on the Am-TiO2/Au Janus micromotor (Fig. 2), indicating the Am-TiO2/Au Janus micromotor shows a negligible enhancement in the photocatalytic activity by Au coupling because it has a high density of defects, which limit the migration distances of the photogenerated electrons. Furthermore, no bubbles can be formed on the crystalline TiO2 (anatase) microspheres (Fig. 3C and D) in the H2O2 fuel under UV irradiation due to the fast diffusion of O2 molecules (diffusion coefficient in water: 2.0 × 10−9 m2 s−1)48 from the convex surfaces,29 indicating the H2O2-sensitized Am-TiO2 has a higher photocatalytic activity for the decomposition of H2O2 than that of the crystalline TiO2. Hence, the fast UV-driven motion of the Am-TiO2/Au Janus micromotor comes from the high photocatalytic activity for H2O2 decomposition of Am-TiO2 and the asymmetric structure.49 The photocatalytic propulsion mechanism of the Am-TiO2/Au Janus micromotor in H2O2 solution is shown in Scheme 1. According to the photocatalytic propulsion mechanism, adjusting UV intensity (I) could change the photon flux on the Am-TiO2/Au Janus micromotors (eqn (4)), and thus the number of the photogenerated holes and electrons. As a result, the generation rate the O2 bubbles and the speed of the micromotors are accordingly modulated. No bubble and motion can be observed for the micromotor when UV is off. When UV intensity increases from 0.1 to 0.8 W cm−2, the speed of the micromotor increases linearly from 21 to 102 μm s−1 with the increasing frequency of the generated bubbles, as shown in ESI-Video 3† and Fig. 4.
 | ||
| Scheme 1 The schematic demonstration of the UV-driven motion of the Am-TiO2/Au Janus micromotor in the H2O2 solution. | ||
The influence of the size of the Am-TiO2/Au Janus micromotor on its light-controlled propulsion in the fuel solution is illustrated in Fig. 5 (corresponding to ESI-Video 4†). The speed of the micromotor firstly increases from 80 to 101 μm s−1 when the size of micromotor increases from 7.5 to 15 μm, and then decreases to 70 and 48 μm s−1 as the size further increases to 22 and 30 μm. This phenomenon could be ascribed to the contrary effect of the size increment on the propulsion of the micromotor. As the size increases, the active sites (exposed surfaces of the Am-TiO2 microsphere) and the photon harvest of the micromotor are increased, leading to the enhanced photocatalytic O2 bubble thrust, as evidenced by the increasing size and frequency of the released O2 bubbles with the increasing size of micromotor from 7.5 to 30 μm (Fig. 5 and ESI-Video 4†). However, on the other hand, the increasing size of the micromotor causes the increasing viscous drag force (Fd) on the micromotor according to Fd = 6πrηv, resulting in the speed reduction. Here, r and v are the radius and speed of the micromotor. η is the dynamic viscosity of water.
 | ||
| Fig. 5 (A) The propulsion of the Am-TiO2/Au Janus micromotor with different sizes under UV irradiation (0.8 W cm−2). Scale bars: 50 μm. (B) Speed of the micromotor versus motor size. | ||
It has been reported that the concentration of the surfactant (Cs) could reduce the surface tension of the H2O2 fuel and subsequently stabilize the released bubbles and reduce their sizes,50 and it thus has a strong impact on the motion of the photoactivated micromotors. The bubble start to grow on one end of the micromotor in the H2O2 fuel with no surfactant contained when the UV irradiation is on. However, the bubble gradually grows to the size of 150 μm and still can not be ejected from the micromotor to propel the micromotor. When Cs increases from 0.01 to 0.05 wt%, the size of the ejected bubbles is reduced from about 65 to 15 μm. Because of their low frequency (0.1 or 0.75 Hz for the micromotor in the H2O2 fuel contains 0.01 or 0.05 wt% Triton-X100, respectively) of the ejected bubbles, the micromotor still can not be propelled (Fig. 6 and ESI-Video 5†). When Cs is further increased from 0.1 to 5 wt%, the size of the ejected bubbles is further reduced to about 10 μm with a increasing ejection frequency of about 10 and 20 Hz, and these ejected bubbles propel the micromotor move forward with an average speed of 18.5 and 37 μm s−1 respectively (Fig. 6 and ESI-Video 5†). The critical micelle concentration (CMC) of aqueous Triton-X100 solution is about 0.02 wt%.51 This suggests that surface tension of the fuel solution is at its lowest value (30.5 mN s−1) when Cs is above 0.02 wt%. Hence, the size and frequency of the released bubbles, as well as the speed of the micromotor are supposed to have a similar value at a given H2O2 fuel concentration when Cs is above 0.02 wt%. However, the micromotor is not even activated at Cs of 0.05 wt%, and its speed still slightly increases from 18.5 to 37 μm s−1 with increasing surfactant concentration from 0.1 to 5 wt% (Fig. 6). It can reasonably be speculated that the surfactant molecules around the micromotor are partially decomposed by the micromotor through photocatalytic reaction, hence changing the surface tension of the local environment and sequentially causing the different motion behaviors when Cs in the fuel is higher than its CMC.
The Am-TiO2/Au Janus micromotors are able to retain efficient bubble propulsion over a wide range of fuel concentrations. ESI-Video 6† and Fig. 7A–F show the propulsion of the micromotor with different fuel concentration (from 0.1 to 8 wt% H2O2). Fig. 7G manifests the influence of fuel concentration on the speed of the micromotors. There is no bubble generation or UV-driven motion for the micromotors when the fuel concentration is lower than 0.1 wt%. When the fuel concentration is higher than 0.5 wt%, the speed increases with the increasing fuel concentration, and exhibit a high average speed of 110 μm s−1 in 8 wt% fuel. It is worthy to notice that the relationship between speed and fuel concentration follows the Michaelis–Menten law (Fig. 7G), suggesting the maximum photocatalytic decomposition rate of H2O2 and the maximum speed of the micromotor is limited by the total active sites of the Am-TiO2/Au Janus microsphere.23
Conclusions
In this work, we have demonstrated a bubble-propelled photoactivated Am-TiO2/Au Janus micromotor by taking advantage of the efficient photocatalytic H2O2 decomposition over the in situ H2O2 sensitized Am-TiO2 under UV irradiation. The motion state and speed of the Am-TiO2/Au micromotors can be reversibly, wirelessly and remotely controlled at will with an ultrafast response rate (less than 0.1 s) by regulating “on/off” switch and intensity of UV irradiation. The quantum efficiency of O2 bubble evolution from photocatalytic decomposition of H2O2 reaches 28%. The power conversion efficiency reaches 1.28 × 10−9 for the photoactivated micromotor during the power conversion process from UV light power, to chemical power and finally to mechanical power. The photoactivated micromotor developed in this work may open up new possibilities for designing highly efficient swimming photocatalysts or adsorbents for water treatment due to their green room-temperature preparation and the intensive mass exchange between the surroundings and the active micromotors.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51303144, 21474078 and 51521001), the Top Talents Lead Cultivation Project and Natural Science Foundation of Hubei Province (2015CFA003 and 2012FFB05101), the Yellow Crane talents plan of Wuhan municipal government, the Fundamental Research Funds for the Central Universities (WUT: 2015-III-060 and 2014-YB-009).Notes and references
- J. Wang,Nanomachines: Fundamentals and Applications, Wiley, 2013 Search PubMed
.
- W. Wang, W. Duan, S. Ahmed, T. E. Mallouk and A. Sen, Nano Today, 2013, 8, 531–554 CrossRef CAS
.
- H. Wang and M. Pumera, Chem. Rev., 2015, 115, 8704–8735 CrossRef CAS PubMed
.
- S. Sánchez, L. Soler and J. Katuri, Angew. Chem., Int. Ed., 2015, 54, 1414–1444 CrossRef PubMed
.
- J. G. S. Moo and M. Pumera, Chem.–Eur. J., 2015, 21, 58–72 CrossRef CAS PubMed
.
- X. Lin, Z. Wu, Y. Wu, M. Xuan and Q. He, Adv. Mater., 2015 DOI:10.1002/adma.201502583
.
- L. Soler and S. Sanchez, Nanoscale, 2014, 6, 7175–7182 RSC
.
- M. Guix, C. C. Mayorga-Martinez and A. Merkoçi, Chem. Rev., 2014, 114, 6285–6322 CrossRef CAS PubMed
.
- W. Gao and J. Wang, Nanoscale, 2014, 6, 10486–10494 RSC
.
- W. Gao and J. Wang, ACS Nano, 2014, 8, 3170–3180 CrossRef CAS PubMed
.
- V. V. Singh, F. Soto, K. Kaufmann and J. Wang, Angew. Chem., Int. Ed., 2015, 54, 6896–6899 CrossRef CAS PubMed
.
- W. Xi, A. A. Solovev, A. N. Ananth, D. H. Gracias, S. Sanchez and O. G. Schmidt, Nanoscale, 2013, 5, 1294–1297 RSC
.
- F. Mou, L. Kong, C. Chen, Z. Chen, L. Xu and J. Guan, Nanoscale, 2015 10.1039/c5nr06774j
.
- Z. G. Wu, Y. J. Wu, W. P. He, X. K. Lin, J. M. Sun and Q. He, Angew. Chem., Int. Ed., 2013, 52, 7000–7003 CrossRef CAS PubMed
.
- F. Mou, C. Chen, Q. Zhong, Y. Yin, H. Ma and J. Guan, ACS Appl. Mater. Interfaces, 2014, 6, 9897–9903 CAS
.
- F. Mou, C. Chen, H. Ma, Y. Yin, Q. Wu and J. Guan, Angew. Chem., Int. Ed., 2013, 52, 7208–7212 CrossRef CAS PubMed
.
- S. Balasubramanian, D. Kagan, C. M. J. Hu, S. Campuzano, M. J. Lobo-Castanon, N. Lim, D. Y. Kang, M. Zimmerman, L. F. Zhang and J. Wang, Angew. Chem., Int. Ed., 2011, 50, 4161–4164 CrossRef CAS PubMed
.
- J. X. Li, W. Gao, R. F. Dong, A. Pei, S. Sattayasamitsathit and J. Wang, Nat. Commun., 2014, 5, 5026 CrossRef CAS PubMed
.
- F. Mou, D. Pan, C. Chen, Y. Gao, L. Xu and J. Guan, Adv. Funct. Mater., 2015, 25, 6173–6181 CrossRef CAS
.
- J. Orozco, G. Cheng, D. Vilela, S. Sattayasamitsathit, R. Vazquez-Duhalt, G. Valdés-Ramírez, O. S. Pak, A. Escarpa, C. Kan and J. Wang, Angew. Chem., Int. Ed., 2013, 52, 13276–13279 CrossRef CAS PubMed
.
- Y.-S. Wang, H. Xia, C. Lv, L. Wang, W.-F. Dong, J. Feng and H.-B. Sun, Nanoscale, 2015, 7, 11951–11955 RSC
.
- J. Wang and K. M. Manesh, Small, 2010, 6, 338–345 CrossRef CAS PubMed
.
- A. A. Solovev, S. Sanchez, M. Pumera, Y. F. Mei and O. G. Schmidt, Adv. Funct. Mater., 2010, 20, 2430–2435 CrossRef CAS
.
- T. R. Kline, W. F. Paxton, T. E. Mallouk and A. Sen, Angew. Chem., Int. Ed., 2005, 44, 744–746 CrossRef CAS PubMed
.
- Y. Y. Hong, M. Diaz, U. M. Cordova-Figueroa and A. Sen, Adv. Funct. Mater., 2010, 20, 1568–1576 CrossRef CAS
.
- S. Giudicatti, S. M. Marz, L. Soler, A. Madani, M. R. Jorgensen, S. Sanchez and O. G. Schmidt, J. Mater. Chem. C, 2014, 2, 5892–5901 RSC
.
- J. Palacci, S. Sacanna, A. P. Steinberg, D. J. Pine and P. M. Chaikin, Science, 2013, 339, 936–940 CrossRef CAS PubMed
.
- J. Palacci, S. Sacanna, A. Vatchinsky, P. M. Chaikin and D. J. Pine, J. Am. Chem. Soc., 2013, 135, 15978–15981 CrossRef CAS PubMed
.
- F. Mou, Y. Li, C. Chen, W. Li, Y. Yin, H. Ma and J. Guan, Small, 2015, 11, 2564–2570 CrossRef CAS PubMed
.
- Z. Liu, J. Li, J. Wang, G. Huang, R. Liu and Y. Mei, Nanoscale, 2013, 5, 1345–1352 RSC
.
- S. Balasubramanian, D. Kagan, K. M. Manesh, P. Calvo-Marzal, G.-U. Flechsig and J. Wang, Small, 2009, 5, 1569–1574 CrossRef CAS PubMed
.
- Z. Wu, X. Lin, Y. Wu, T. Si, J. Sun and Q. He, ACS Nano, 2014, 8, 6097–6105 CrossRef CAS PubMed
.
- T. L. Xu, F. Soto, W. Gao, V. Garcia-Gradilla, J. X. Li, X. J. Zhang and J. Wang, J. Am. Chem. Soc., 2014, 136, 8552–8555 CrossRef CAS PubMed
.
- A. A. Solovev, E. J. Smith, C. C. B. Bufon, S. Sanchez and O. G. Schmidt, Angew. Chem., Int. Ed., 2011, 50, 10875–10878 CrossRef CAS PubMed
.
- P. Calvo-Marzal, K. M. Manesh, D. Kagan, S. Balasubramanian, M. Cardona, G.-U. Flechsig, J. Posner and J. Wang, Chem. Commun., 2009, 30, 4509–4511 RSC
.
- Y. Li, T. Sasaki, Y. Shimizu and N. Koshizaki, J. Am. Chem. Soc., 2008, 130, 14755–14762 CrossRef CAS PubMed
.
- Y. Shiraishi, N. Saito and T. Hirai, J. Am. Chem. Soc., 2005, 127, 12820–12822 CrossRef CAS PubMed
.
- T. Xia, W. Zhang, J. B. Murowchick, G. Liu and X. Chen, Adv. Energy Mater., 2013, 3, 1516–1523 CrossRef CAS
.
- J. Zou, J. Gao and F. Xie, J. Alloys Compd., 2010, 497, 420–427 CrossRef CAS
.
- J. Zou and J. Gao, J. Hazard. Mater., 2011, 185, 710–716 CrossRef CAS PubMed
.
- W. Gao, X. Feng, A. Pei, Y. Gu, J. Li and J. Wang, Nanoscale, 2013, 5, 4696–4700 RSC
.
- W. Gao, A. Pei and J. Wang, ACS Nano, 2012, 6, 8432–8438 CrossRef CAS PubMed
.
- W. Gao, M. D'Agostino, V. Garcia-Gradilla, J. Orozco and J. Wang, Small, 2013, 9, 467–471 CrossRef CAS PubMed
.
- H. Yan, J. Yang, G. Ma, G. Wu, X. Zong, Z. Lei, J. Shi and C. Li, J. Catal., 2009, 266, 165–168 CrossRef CAS
.
- M. Manjare, B. Yang and Y. P. Zhao, J. Phys. Chem. C, 2013, 117, 4657–4665 CAS
.
- W. Wang, T.-Y. Chiang, D. Velegol and T. E. Mallouk, J. Am. Chem. Soc., 2013, 135, 10557–10565 CrossRef CAS PubMed
.
- P. D. Tran, L. H. Wong, J. Barber and J. S. C. Loo, Energy Environ. Sci., 2012, 5, 5902–5918 CAS
.
- J.-U. Kreft, C. Picioreanu, J. W. T. Wimpenny and M. C. M. van Loosdrecht, Microbiology, 2001, 147, 2897–2912 CrossRef CAS PubMed
.
- F. Mou, C. Chen, J. Guan, D.-R. Chen and H. Jing, Nanoscale, 2013, 5, 2055–2064 RSC
.
- W. Huang, M. Manjare and Y. Zhao, J. Phys. Chem. C, 2013, 117, 21590–21596 CAS
.
- R. M. Manglik, V. M. Wasekar and J. Zhang, Exp. Therm. Fluid Sci., 2001, 25, 55–64 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figures and videos. See DOI: 10.1039/c5ra26798f |
| This journal is © The Royal Society of Chemistry 2016 |