Surface oxygen vacancy assisted electron transfer and shuttling for enhanced photocatalytic activity of a Z-scheme CeO2–AgI nanocomposite†
Abstract
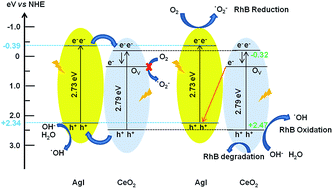
Surface-oxygen-vacancy-promoted Z-scheme CeO2–AgI heterostructured photocatalysts were successfully fabricated via a hydrothermal route combined with a precipitation process. Surface oxygen vacancies were formed on the synthesized CeO2–AgI photocatalyst, as determined by X-ray photoelectron spectroscopy. These oxygen vacancies could extend the lifetime of the charge carriers and enhance the photocatalytic activity of these catalysts for rhodamine B (RhB) dye degradation. Among the as-synthesized photocatalysts, the 20 wt% CeO2–AgI (CA-2) nanocomposite demonstrated the highest photocatalytic activity towards the degradation of RhB with 3.28- and 29.8-fold higher activity than pure AgI and CeO2 nanostructures, respectively. In addition, to ensure the visible light photocatalytic activity of the CeO2–AgI nanocomposite, decomposition studies were performed using a colorless substrate such as phenol. The mechanism for the enhanced photocatalytic performance of the CeO2–AgI photocatalyst is proposed to be based on efficient separation of photogenerated electron–hole pairs through a Z-scheme system, in which oxygen vacancy states promote charge separation. Experiments using scavengers of reactive species combined with photoluminescence analysis provide significant evidence for the oxygen-vacancy-mediated Z-scheme mechanism of the photocatalyst. Moreover, the as-prepared oxygen-deficient CeO2–AgI photocatalysts exhibited excellent cycling stability.

 Please wait while we load your content...
Please wait while we load your content...